Abstract
Background
We aim to identify the multifaceted risk factors that can affect the development of severe radiation pneumonitis (RP) in patients with non-small cell lung cancer (NSCLC) treated with curative high-dose radiotherapy with or without concurrent chemotherapy.
Methods
We retrospectively reviewed the medical records of 175 patients with stage-I-III NSCLC treated with curative thoracic X-ray radiotherapy at the Korea University Guro Hospital between June 2019 and June 2022. Treatment-related complications were evaluated using the Common Terminology Criteria for Adverse Events (version 4.03).
Results
The median follow-up duration was 15 months (range: 3–47 months). Idiopathic pulmonary fibrosis (IPF) as an underlying lung disease (P < 0.001) and clinical stage, regarded as the concurrent use of chemotherapy (P = 0.009), were associated with a high rate of severe RP. In multivariate analyses adjusting confounding variables, the presence of IPF as an underlying disease was significantly associated with severe RP (odds ratio [95% confidence interval] = 48.4 [9.09–347]; P < 0.001). In a subgroup analysis of stage-I-II NSCLC, the incidence of severe RP in the control, chronic obstructive pulmonary disease (COPD), and IPF groups was 3.2%, 4.3%, and 42.9%, respectively (P < 0.001). The incidence of severe RP was 15.2%, 10.7%, and 75.0% in the control, COPD, and IPF groups, respectively (P < 0.001) in the stage-III NSCLC group.
Conclusions
This study revealed that IPF as an underlying lung disease and the concurrent use of chemotherapy are associated with a high rate of severe RP. In contrast, COPD did not increase the risk of pulmonary toxicity after receiving curative high-dose radiotherapy.
Similar content being viewed by others
Background
Radiation pneumonitis (RP), an inflammatory reaction in the lungs due to radiotherapy for the thoracic organs, most commonly develops within 3–4 months after finishing radiotherapy; however, it can occur up to 6 months after radiotherapy and can lead to permanent scarring of the lung tissue, known as pulmonary fibrosis. In some cases, RP alone can affect morbidity and mortality rates in patients with lung cancer.
Although studies on various factors affecting the development of severe RP have been conducted [1,2,3,4,5], a consensus has not yet been reached. In addition, remarkable advances in radiotherapy techniques have not been sufficiently demonstrated. Several studies have suggested that underlying lung diseases can affect the incidence of severe RP [6,7,8,9,10,11,12,13,14,15]; of which, idiopathic pulmonary fibrosis (IPF) has a higher incidence of RP than that of other diseases [6, 7, 16, 17]. It can lead to rapid disease deterioration after radiotherapy and substantially contributes to the poor prognosis of these patients. However, the effect of chronic obstructive pulmonary disease (COPD) on severe RP after curative radiotherapy has not been fully investigated [9,10,11].
In this context, we aim to identify the multifaceted risk factors that can affect the development of severe RP in patients with non-small cell lung cancer (NSCLC) treated with curative high-dose radiotherapy with or without concurrent chemotherapy, with focus on underlying pulmonary disease.
Methods
Patients
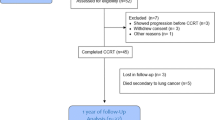
After obtaining the Institutional Review Board approval (no. 2023GR0216), we retrospectively reviewed the medical records of 175 patients with stage-I-III NSCLC treated with curative high-dose thoracic X-ray radiotherapy at Korea University Guro Hospital between June 2019 and June 2022. Patients who did not undergo a pulmonary function test (PFT) during the staging workup process or those who did not complete radiotherapy were excluded.
Diagnostic scheme for lung cancer and underlying lung disease
Tumor assessment comprised obtaining PFT, chest radiographs, computed tomography (CT) scans of the chest and upper abdomen, whole-body 18F-fluorodeoxyglucose positron emission tomography with CT scans, and magnetic resonance scans of the brain as a routine staging work-up. The PFT included (1) forced expiratory volume in 1 s (FEV1), (2) forced vital capacity (FVC), (3) ratio of the two volumes (FEV1/ FVC), and (4) diffusing capacity of the lungs for carbon monoxide, before treatment. All diagnoses of underlying lung diseases, such as COPD and IPF, were confirmed by experienced pulmonologists (J.H.C.). Treatment-related complications were evaluated using the Common Terminology Criteria for Adverse Events (version 4.03).
Treatment scheme
The planned total dose and fractions differed according to the location of lung lesions. Based on institutional protocol, stereotactic ablative radiation therapy (SABR) with a total dose of 60 Gy in four fractions was administered to patients with small-sized (≤ 4 cm) NSCLC and peripherally located tumors. For patients who received intensity-modulated radiation therapy (IMRT), two different dose-fractionation schedules were planned for delivering 60 Gy in 20 fractions in the radiotherapy alone group and 66/60 Gy in 30 fractions in the concurrent chemoradiotherapy group via the simultaneous integrated boost technique. According to the prescription guidelines, we delivered at least 97% of the prescribed dose to 95% of the PTV. The minimum and maximum doses to 1 cc of the PTV were 95% and 107%, respectively. The percentage of the lung volume that received ≥ 5 Gy (V5) and 20 Gy (V20) was maintained at ≤ 65% and 35%, respectively, and the mean lung dose (MLD) was ≤ 20 Gy.
Statistical analyses
Overall survival (OS) was defined as the time from the start of radiotherapy until the date of death due to any cause or the latest documented follow-up visit. The 2-year OS rate was calculated using the Kaplan–Meier method and was compared using the log-rank test. To compare clinical characteristics according to the occurrence of severe lung toxicity, we used Chi-square or Fisher’s exact tests to assess categorical variables and used an independent-sample t-test to assess continuous variables. Multivariate generalized linear regression analyses were performed to assess associations between variables and RP outcomes. Statistical significance was set at p < 0.05 in two-tailed tests. Statistical analyses were performed using the IBM SPSS Statistics for Windows (version 24.0; IBM Corp., Armonk, NY, USA), and R statistics software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
The patients’ clinical characteristics are summarized in Table 1. The median age of the study population was 76 years (range: 38–93 years). Most patients were men (76.0%) and current or ex-smokers (53.7%). Of the 175 patients, 93 (53.1%) were diagnosed with stage-I or -II cancer and the remaining 82 (46.9%) with stage-III cancer. Excluding patients with no underlying lung disease, 51 (51/175, 29.1%) were diagnosed with COPD and 15 (15/175, 8.6%) with IPF. Regarding the radiotherapy technique, 36 patients were treated with SABR (20.6%), and the remaining 139 were treated with IMRT (79.4%).
Patterns of failure and survival outcomes
The median follow-up duration was 15 months (range: 3–47 months). Distant metastasis was the most common recurrent type in both stage-I and -II (31/93 patients, 33.3%) and stage-III NSCLC groups (27/82 patients, 32.9%) (Fig. 1). In terms of radiotherapy, in-field local recurrence occurred in four patients in each group. The 2-year OS rate was 72.8% in patients with stage-I or -II cancer, and 70.7% in patients with stage-III cancer.
Treatment-related complications
The clinical characteristics, including patient, tumor, and chemo- and radiotherapy-related factors, according to the occurrence of severe RP are summarized in Table 2. No statistically significant differences were noted in sex, smoking status, histology, or pretreatment PFT values. Regarding the radiotherapy planning parameters, tumor volume and lung parameters, such as MLD, V5, V10, and V20, were similar between the two groups. However, IPF as an underlying lung disease (P < 0.001) and the clinical stage, based on the concurrent use of chemotherapy (P = 0.009), were associated with a high rate of severe RP. In multivariate generalized linear regression analysis, the presence of IPF as an underlying pulmonary disease was significantly associated with severe RP, and retained after application of backward elimination method (Table 3).
Regarding treatment-related lung toxicity, the incidence of severe RP was 12.6% (22/175). Specifically, the incidence of severe RP in the control, COPD, and IPF groups was 8.3% (9/109), 7.8% (4/51), and 60.0% (9/15), respectively (P < 0.001) (Table 4). In the subgroup analysis of stage-I-II NSCLC, the incidence of severe RP in the control, COPD, and IPF groups was 3.2%, 4.3%, and 42.9%, respectively (P < 0.001). The incidence of severe RP was 15.2%, 10.7%, and 75.0% in the control, COPD, and IPF groups, respectively (P < 0.001) in the stage-III NSCLC group. The incidence of severe RP was similar between the control and COPD groups in patients with stage-I-II and -III NSCLC. Conversely, IPF was significantly associated with a high incidence of severe RP in a cohort of patients with stage-I–III NSCLC.
Discussion
Severe RP is one of the most common treatment-related toxicities after receiving curative high-dose radiotherapy and can affect the mortality rate of patients with lung cancer. Although previous studies on various factors affecting the occurrence of severe RP have been conducted [1,2,3, 18, 19], no consensus has been yet reached. In addition, the technical aspects of radiotherapy based on the latest knowledge may not be fully reflected. The following previously known risk factors are known for each parameter: (1) patient factors, including male sex, smoking history, underlying lung disease, and poor lung function; (2) tumor factors, such as tumor size and location; (3) treatment factors, such as concurrent use of chemotherapy; and (4) dose-volume histogram-based dosimetric parameters, such as V5, V20, and MLD, which are known as predictive markers for severe RP, despite some controversies.
In terms of underlying lung disease, previous studies reported that patients with interstitial lung disease (ILD) are more susceptible to develo** severe RP after receiving high-dose radiotherapy [6,7,8, 19]. However, ILD is not a single disease, but rather a broad group of diseases that mainly lead to problems in the lung parenchyma. Some studies, confined to IPF, reported a high incidence of severe RP after radiotherapy in these patient groups [16, 17]. However, IPF itself is a rare disease, and most studies have limitations regarding the small number of patients. Moreover, the impact of COPD on the risk of severe RP development after curative radiotherapy has not been fully investigated, and there are conflicting data [9,10,11]. Some studies have shown that COPD is associated with a high risk of RP, whereas other studies reported that RP is relatively mild in patients with severe COPD. Regarding the radiotherapy planning parameters, there are limitations in terms of interpreting the results of existing studies, as radiotherapy treatment technology has recently switched from the previous 3-dimensional conformal radiotherapy to the more advanced IMRT or SABR [4, 5].
In this context, we aimed to identify the multifaceted prognostic factors that can affect the development of severe RP in patients with NSCLC treated with curative high-dose radiotherapy with or without concurrent chemotherapy. In the current study, clinical characteristics were divided into patient, tumor, and treatment-related factors. No statistically significant differences were detected in sex, smoking status, histology, or pretreatment PFT values. With respect to the radiotherapy planning parameters, although the tumor volume was somewhat larger in the severe RP event group, the dosimetric parameters of the IMRT and SABR treatment plans, such as MLD, V5, and V20, showed similar values between the groups. However, IPF as an underlying lung disease (P < 0.001) and the concurrent use of chemotherapy (P = 0.009) were associated with a high rate of severe RP, which is consistent with previous results. As a result of the analysis of specific underlying lung diseases, the incidence of severe RP was similar between the control and COPD groups in both the early- (3.2% vs. 4.3%) and locally advanced-stage (15.2% vs. 10.7%) NSCLC. Conversely, IPF was significantly associated with severe RP development in patients at all stages of NSCLC (early stage, 42.9%; locally advanced stage, 75.0%).
The current study had several limitations. First, it was a retrospective analysis, and there might have been selection bias. Second, the sample size was too small to show a statistically significant difference between the two groups.
Conclusions
This study showed that IPF as an underlying lung disease and the concurrent use of chemotherapy were associated with a high rate of severe RP. Conversely, the presence of COPD did not increase the risk of pulmonary toxicity after receiving curative high-dose radiotherapy.
Availability of data and materials
The datasets used and/or analyzed in the current study can be obtained from the corresponding author upon reasonable request.
Abbreviations
- RP:
-
Radiation pneumonitis
- NSCLC:
-
Non-small cell lung cancer
- IPF:
-
Idiopathic pulmonary fibrosis
- COPD:
-
Chronic obstructive pulmonary disease
- PFT:
-
Pulmonary function test
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- SABR:
-
Stereotactic ablative radiation therapy
- IMRT:
-
Intensity-modulated radiation therapy
- MLD:
-
Mean lung dose
- OS:
-
Overall survival
- ILD:
-
Interstitial lung disease
References
Claude L, Pérol D, Ginestet C, Falchero L, Arpin D, Vincent M, et al. A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: clinical and dosimetric factors analysis. Radiother Oncol. 2004;71(2):175–81.
Shi A, Zhu G, Wu H, Yu R, Li F, Xu B. Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat Oncol. 2010;5:35.
Park YH, Kim JS. Predictors of radiation pneumonitis and pulmonary function changes after concurrent chemoradiotherapy of non-small cell lung cancer. Radiat Oncol J. 2013;31(1):34–40.
Leprieur EG, Fernandez D, Chatellier G, Klotz S, Giraud P, Durdux C. Acute radiation pneumonitis after conformational radiotherapy for nonsmall cell lung cancer: clinical, dosimetric, and associated-treatment risk factors. J Cancer Res Ther. 2013;9(3):447–51.
Baker R, Han G, Sarangkasiri S, DeMarco M, Turke C, Stevens CW, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys. 2013;85(1):190–5.
Yamashita H, Kobayashi-Shibata S, Terahara A, Okuma K, Haga A, Wakui R, et al. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat Oncol. 2010;5:32.
Yamaguchi S, Ohguri T, Ide S, Aoki T, Imada H, Yahara K, et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis. Lung Cancer. 2013;82(2):260–5.
Lee YH, Kim YS, Lee SN, Lee HC, Oh SJ, Kim SJ, et al. Interstitial lung change in pre-radiation therapy computed tomography is a risk factor for severe radiation pneumonitis. Cancer Res Treat. 2015;47(4):676–86.
Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol. 2003;67(3):275–83.
Takeda A, Kunieda E, Ohashi T, Aoki Y, Oku Y, Enomoto T, et al. Severe COPD is correlated with mild radiation pneumonitis following stereotactic body radiotherapy. Chest. 2012;141(4):858–66.
Zhou Z, Song X, Wu A, Liu H, Wu H, Wu Q, et al. Pulmonary emphysema is a risk factor for radiation pneumonitis in NSCLC patients with squamous cell carcinoma after thoracic radiation therapy. Sci Rep. 2017;7(1):2748.
Kanaji N, Tadokoro A, Kita N, Murota M, Ishii T, Takagi T, et al. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J Cancer Res Clin Oncol. 2016;142(8):1855–65.
Lee T, Park JY, Lee HY, Cho YJ, Yoon HI, Lee JH, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med. 2014;108(10):1549–55.
Moon SW, Park MS, Kim YS, Jang J, Lee JH, Lee CT, et al. Combined pulmonary fibrosis and emphysema and idiopathic pulmonary fibrosis in non-small cell lung cancer: impact on survival and acute exacerbation. BMC Pulm Med. 2019;19(1):177.
Tomassetti S, Gurioli C, Ryu JH, Decker PA, Ravaglia C, Tantalocco P, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015;147(1):157–64.
Kim H, Pyo H, Noh JM, Lee W, Park B, Park HY, et al. Preliminary result of definitive radiotherapy in patients with non-small cell lung cancer who have underlying idiopathic pulmonary fibrosis: comparison between X-ray and proton therapy. Radiat Oncol. 2019;14(1):19.
Kim H, Yoo H, Pyo H, Ahn YC, Noh JM, Ju SG, et al. Impact of underlying pulmonary diseases on treatment outcomes in early-stage non-small cell lung cancer treated with definitive radiotherapy. Int J Chron Obstruct Pulmon Dis. 2019;14:2273–81.
Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48(1):89–94.
Li F, Liu H, Wu H, Liang S, Xu Y. Risk factors for radiation pneumonitis in lung cancer patients with subclinical interstitial lung disease after thoracic radiation therapy. Radiat Oncol. 2021;16(1):70.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Research Foundation of Korea grant, funded by the Korean Government (MSIT) (No. 2022R1A2C109170011).
This work was supported by the Korea University Guro Hospital (KOREA RESEARCH-DRIVEN HOSPITAL) and a grant funded by the Korea University Medicine (No. K2313801).
Author information
Authors and Affiliations
Contributions
HKK and DSY conceived and designed the study. HKK, JEH, SMK, and JHC analyzed and interpreted the patient data. HKK and JEH drafted the manuscript. DSY was involved in critically revising the manuscript for important intellectual content. All the authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Korea University Medical Center, Institutional Review Board (no. 2023GR0216), and the requirement for an informed consent to participate was waived owing to the retrospective nature of the study by Institutional Review Board review committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, H., Hwang, J., Kim, S.M. et al. Risk factor analysis of the development of severe radiation pneumonitis in patients with non-small cell lung cancer treated with curative radiotherapy, with focus on underlying pulmonary disease. BMC Cancer 23, 992 (2023). https://doi.org/10.1186/s12885-023-11520-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11520-y