Abstract
Objective
Antibody-drug conjugates (ADCs) that target human epidermal growth factor receptor 2 (HER2) are leading a new era of targeted cancer therapy. These drugs have also been associated with several fatal adverse events, such as pneumonia, interstitial lung disease, and infection. We performed a meta-analysis of randomized controlled trials (RCTs) to estimate the incidence and risk of fatal adverse events in cancer patients treated with HER2-targeted ADCs.
Methods
We performed a systematic search in Embase, PubMed, Web of Science, and Scopus databases from inception to February 1, 2022, and the last search was updated to July 1, 2023. The eligible studies for inclusion in our analysis were limited to RCTs of HER2-targeted ADCs that were approved by the US Food and Drug Administration and examined on cancer patients with available data on fatal adverse events. The protocol for this study was registered in PROSPERO (No. CRD42022331627).
Results
Fifteen studies (13 RCTs) involving 7,277 patients were finally included for meta-analysis. Of these patients, 4,246 received HER2-targeted ADCs and 3,481 received the control treatment. The data were combined using Bayesian hierarchical modeling, which allowed for the estimation of the mean incidence of fatal adverse events to be 0.78% (95% CrI: 0.28-1.37%, τ = 0.006) for the patients treated with HER2-targeted ADCs. The relative risk was 0.80 (95% CrI, 0.5–1.26, τ = 0.17) compared to control patients. Among 43 reported deaths caused by HER2-targeted ADCs, the most common fatal adverse event was respiratory toxicity, including pneumonia, pneumonitis, and interstitial lung disease. On subgroup analysis, no difference in the risk of fatal adverse events was found between different HER2-targeted ADCs or cancer types.
Conclusion
Our findings suggest that the risk of fatal adverse events with HER2-targeted ADCs may be lower compared to standard control therapies in cancer patients, and there is no significant difference in risk observed between different HER2-targeted ADCs or cancer types. However, the most common fatal adverse event was respiratory toxicity, suggesting that cancer patients who use the above drugs should strengthen respiratory system monitoring and take preventive measures in some severe cases.
Similar content being viewed by others
Introduction
Human epidermal growth factor receptor 2 (HER2) plays a crucial role in tumor growth, invasion, and development [1, 2]. Extensive studies have shown that HER2 expression is closely related to the occurrence of various tumors and is one of the most important targets for develo** anticancer therapies [3, 4]. Antibody-drug conjugate (ADC), which is composed of a monoclonal antibody linked to a cytotoxic agent, is revolutionizing targeted cancer therapy [5]. HER2, as a classic tumor target, has become an ideal target for the development of ADC drugs due to its high specific expression in tumor tissue and its high efficiency in mediating the endocytosis of ADC drugs. To date, the US Food and Drug Administration (FDA) has approved two ADC drugs (T-DM1 and T-DXd), and over 60 HER2-targeted ADC candidates are currently undergoing clinical trials [6].
HER2-targeted ADCs have shown excellent efficacy and have had their indications expanded on the strength of their ingenious design of the molecular structure that delivers cytotoxic drugs specifically to cancer cells [7,8,9]. However, fatal adverse events have been reported with HER2-targeted ADCs due to undesired uptake in healthy cells. Some of these adverse events can be life-threatening, such as pneumonitis, hematotoxicity, cardiotoxicity, and hepatotoxicity [10,11,12]. The occurrence of fatal adverse events is difficult to avoid during anticancer treatments and causes great harm to patients and their families. In order to improve the treatment compliance of patients and ensure that patients can continue to benefit from the treatment of HER2-targeted ADCs, it is particularly important to clearly understand the profile of fatal adverse events and timely develop management measures. And the analysis of fatal adverse events will help to improve the guidelines and provide guidance for better guiding the clinical application of HER2-targeted ADC drugs.
Multiple clinical trials have been concerned with the fatal adverse events caused by HER2-targeted ADCs, but the limited number of patients in each trial has left the overall incidence and risk of such events unclear. Therefore, in this study, we conducted a systematic review and meta-analysis of the mortality profile of HER2-targeted ADCs. Utilizing a Bayesian hierarchical modeling approach, we quantitatively combined data from randomized controlled trials (RCTs) to address the incidence and risk of fatal adverse events in cancer patients treated with HER2-targeted ADCs. We aim to provide clinicians with a reference to use HER2-targeted ADCs appropriately and manage potential fatal adverse events related to these drugs.
Methods
Search methods and study selection
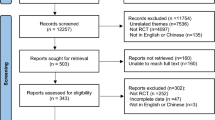
The present systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline (eTable 1 in the Supplement) [13, 14]. The protocol was registered in PROSPERO (No. CRD42022331627). We systematically searched the PubMed, Embase, Web of Science, and Scopus databases from inception to February 1, 2022, and updated the last search to July 1, 2023. The keywords, including “HER2 ADC”, “trastuzumab emtansine (T-DM1)”, and “trastuzumab deruxtecan (T-Dxd)”, are employed and full search strategy is detailed in eTable 2 in Supplement. We also manually screened reference lists from relevant review articles to supplement the search. This study excluded non-randomized trials, editorials, correspondences, and reviews. We included only prospective RCTs of HER2-targeted ADC agents in the treatment of cancer patients. The inclusion criteria were based on the PICO-framework. In detail, Population (P): cancer patients; Intervention (I): treatments by HER2-targeted ADC agents. Comparison (C): The other therapeutics. Outcomes (O): any fatal adverse events. When publications reported the same trial, the most recent one was included. Two reviewers (JY ** fatal adverse events with the HER2-targeted ADCs across the studies was RR = 0.80 (95% CrI, 0.5–1.26, τ = 0.17) (Fig. 3). When stratified by each used HER2-targeted ADC, the incidence was 0.82% (95% CrI, 0.22–1.55%, τ = 0.007) for T-DM1 and 0.78%(95%CrI,0–2.89%, τ = 0.015) for T-DXd. As for the different cancer types, the incidences of the fatal adverse event caused by HER2-targeted ADCs in breast cancer patients and gastric cancer patients were determined as 0.66% (95% CrI, 0.18–1.24%, τ = 0.006) and 2.01%(95%CrI,0–4.41%, τ = 0.089), respectively. The subgroup analyses based on HER2-targeted ADC drugs and cancer types did not reveal any meaningful differences. Figure 4 demonstrates the overall and stratified analysis.
Forest plots of the model posteriors for overall risk of mortality caused by HER2-targeted ADCs. Forest plots from the 15 studies display the median and CrI of posterior µ and θk+1 estimates. Quoted study estimates yi and shrinkage estimate θi for i = 1–15 are also shown. The posterior median µ is shown as exponentiated (standard) linear risk ratios, wherein a null effect equals 1. CrI = credible interval
Sensitivity analysis and publication bias
We conducted a sensitivity analysis by using various prior distributions for the between-study variance (τ) and presented the results in eTable 4 in the Supplement. The sensitivity analysis indicated that the RR of fatal adverse events with HER2-targeted ADCs remained consistent, supporting the robustness of our estimated effect size using Bayesian hierarchical modeling. Additionally, we assessed the possibility of publication bias using a classic funnel plot [28]. The funnel plot (eFigure 1 in Supplement) indicated significant asymmetry in fatal adverse events, indicating no publication bias in the included trials.
Discussion
Based on the 13 clinical RCTs, including 4,246 patients, the pooled incidence of fatal adverse events in patients treated with a HER2-targeted ADC was 0.078% compared with 0.095% in patients from control arms, and this risk was lower but not significantly than that with the control arm (RR = 0.8; 95% CrI, 0.5–1.26, τ = 0.17). These findings suggest that HER2-targeted ADCs may be a relatively safe and comparable alternative to standard conventional therapies for cancer patients, making them promising novel therapeutic options in clinical settings.
As an emerging biopharmaceutical drug, the HER2-targeted ADCs have provided promising alternative ways to fight against cancer [29]. However, the information on fatal adverse events associated with HER2-targeted ADCs remains unclear. Cancer therapy using HER2-targeted ADCs is a double-edged sword. While focusing on efficacy, we should also pay close attention to the adverse event caused by drugs, especially the fatal ones, since it severely impacts patients and their families. Therefore, it is necessary to investigate the incidence of fatal adverse events to properly evaluate the benefit-risk ratio and make decisions in the oncology clinic. Our study showed that treatment with HER2-targeted ADCs results in 0.078% of patients dying due to adverse effects of ADC treatment alone. It also revealed a lower risk of fatal adverse events compared to the other standard therapies (RR = 0.8). These data should be essential in considering whether to use HER2-targeted ADCs treatment.
Among 43 reported deaths, the most common cause of death caused by HER2-targeted ADCs was respiratory toxicity, including six pneumonia, three pneumonitis, and one each of interstitial lung disease, pulmonary embolism, pneumonia aspiration, lung infection, bronchopneumonia, dyspnea, and atypical pneumonia. Besides, hematologic toxicity, infection, and hepatic toxicity accounted for the other leading cause of death. This meta-analysis demonstrates that the risk of fatal adverse events with HER2-targeted ADCs is comparable to conventional anticancer therapy. Therefore, it is crucial to closely monitor patients receiving HER2-targeted ADCs for symptoms related to the respiratory system, infection, and liver functions. Early recognition and management of toxic effects, including prompt initiation of dose reduction and other modulating agents like glucocorticoids, are essential for preventing fatalities.
Previous meta-analyses have estimated the incidence and risk of adverse events associated with T-DM1, but they did not analyze fatal events [30,31,32,33]. There was also a meta-analysis that investigated the incidence of general adverse events related to antibody-drug conjugates in all clinical trials, including lymphopenia, nausea, neutropenia, peripheral neuropathy, and blurred vision [34]. However, our study concentrated on fatal adverse events instead of general adverse events and also focused on the HER2-targeted ADCs, which represent the most common subclass of ADCs. Our meta-analysis is the largest to date, including 4,246 patients from high-quality RCTs, and provides a summary of HER2-targeted ADCs-related fatal adverse events in cancer patients. The results revealed that the toxicities of the respiratory system and myelosuppression attributed to the leading cause of death among patients receiving HER2-targeted ADCs. The reasonable interpretation of that was the high amounts of FcγR expressed in alveolar macrophages and myeloid cells and Fc-mediated non-specific uptake of HER2-targeted ADCs might contribute to these fatal adverse events [35, 36]. Consequently, the next generation of HER2-targeted ADCs could consider optimizing the Fc fragments of the antibody part of ADCs, such as an increase in serum stability or improvement of binding specificity and affinity.
However, some limitations could be improved in our study. Firstly, our study relied on study-level data, and individual patient-level confounding factors could not be thoroughly assessed or included in the analysis. Secondly, since the primary outcomes of included RCTs were focused on the efficacy of HER2-targeted ADCs, the fatal adverse events were reported through different investigators and institutions, which might introduce potential bias in the assessment of whether fatal adverse events were associated with the treatment of HER2-targeted ADCs. Thirdly, probably because of the small sample size and potential reasons related to cancer, our analysis showed no evident risk difference between HER2-targeted ADCs and control-arm therapies. Finally, due to the scarcity of studies involving T-DXd in non-breast cancer patients, the pre-defined stratification factors (i.e., drug and cancer types) were insufficient to detect significant differences in the risk associated with distinct HER2-ADCs or cancer types.
Conclusions
Based on our systematic review and Bayesian meta-analysis, this study reveals the incidence and risk of fatal adverse events associated with HER2-targeted ADCs in cancer patients involved in RCTs. The results indicate that the risk of fatal adverse events with HER2-targeted ADCs may be lower compared to standard control therapies in cancer patients. Moreover, our study found no significant difference in the risk of fatal adverse events between different HER2-targeted ADCs or cancer types. However, the most common fatal adverse event was respiratory toxicity, suggesting that cancer patients who use the above drugs should strengthen respiratory system monitoring and take preventive measures in some severe cases.
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files.
References
Korkaya H, et al. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27(47):6120–30.
Appert-Collin A, et al. Role of ErbB receptors in cancer cell migration and invasion. Front Pharmacol. 2015;6:283.
Raymond E, Faivre S, Armand JP. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs. 2000;60(1):15–23.
Kamath S, Buolamwini JK. Targeting EGFR and HER-2 receptor tyrosine kinases for cancer drug discovery and development. Med Res Rev. 2006;26(5):569–94.
Fu Z, et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Therapy. 2022;7(1):1–25.
Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discovery, 2022: p. 1–26.
Siena S, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22(6):779–89.
Shitara K, et al. Trastuzumab Deruxtecan in previously treated HER2-Positive gastric Cancer. N Engl J Med. 2020;382(25):2419–30.
Li BT, et al. Trastuzumab Deruxtecan in HER2-Mutant non-small-cell Lung Cancer. N Engl J Med. 2022;386(3):241–51.
Modi S, et al. Trastuzumab Deruxtecan in previously treated HER2-Low advanced breast Cancer. N Engl J Med. 2022;387(1):9–20.
Krop IE, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743–54.
Emens LA, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21(10):1283–95.
Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst reviews. 2015;4(1):1–9.
Shamseer L, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647.
Béliveau A, et al. BUGSnet: an R package to facilitate the conduct and reporting of bayesian network Meta-analyses. BMC Med Res Methodol. 2019;19(1):1–13.
Liu W, et al. Vitamin D status in mainland of China: a systematic review and meta-analysis. EClinicalMedicine. 2021;38:101017.
Sterne J, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Perez EA, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2–positive advanced breast cancer: final results from MARIANNE. Cancer. 2019;125(22):3974–84.
Krop IE, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–99.
Cortés J, et al. Efficacy and safety of Trastuzumab Emtansine Plus Capecitabine vs Trastuzumab Emtansine alone in patients with previously treated ERBB2 (HER2)-Positive metastatic breast Cancer: a phase 1 and Randomized Phase 2 Trial. JAMA Oncol. 2020;6(8):1203–9.
Cortes J, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for breast Cancer. N Engl J Med. 2022;386(12):1142–54.
Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91.
von Minckwitz G, et al. Trastuzumab Emtansine for residual invasive HER2-Positive breast Cancer. N Engl J Med. 2019;380(7):617–28.
Hurvitz SA, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31(9):1157–63.
Thuss-Patience PC, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53.
Tolaney SM, et al. Adjuvant trastuzumab Emtansine Versus Paclitaxel in Combination with Trastuzumab for Stage I HER2-Positive breast Cancer (ATEMPT): a Randomized Clinical Trial. J Clin Oncol. 2021;39(21):2375–85.
Chiradoni Thungappa S, et al. Comparison of the efficacy, Safety, Pharmacokinetic and Immunogenicity of UJVIRA (ZRC-3256, Trastuzumab Emtansine) with the Kadcyla (Trastuzumab Emtansine) in the treatment of HER2-Positive metastatic breast Cancer: a randomized, Open-Label, Multicenter Study in India. Clin Breast Cancer. 2022;22(4):300–7.
Sterne JA, Harbord RM. Funnel plots in meta-analysis. Stata J. 2004;4(2):127–41.
Menderes G, et al. Mechanisms of resistance to HER2-targeted therapies in HER2-amplified uterine serous carcinoma, and strategies to overcome it. Discov Med. 2018;26(141):39–50.
Liu K et al. Incidence and risk of severe adverse events associated with trastuzumab emtansine (T-DM1) in the treatment of breast cancer: an up-to-date systematic review and meta-analysis of randomized controlled clinical trials. Expert Rev Clin Pharmacol, 2022: p. 1–8.
Jahan N et al. Relative risk of peripheral neuropathy with ado-trastuzumab emtansine (T-DM1) compared to taxane-based regimens in human epidermal growth factor receptor 2 (HER2)-positive cancers: a systematic review and meta-analysis. Cureus, 2021. 13(5).
Ma B, et al. Clinical efficacy and safety of T-DM1 for patients with HER2-positive breast cancer. OncoTargets and therapy. 2016;9:959.
Fu Z et al. Treatment-related adverse events associated with HER2-Targeted antibody-drug conjugates in clinical trials: A systematic review and meta-analysis eClinicalMedicine, 2022: p. 101795.
Zhu Y, et al. Treatment-related adverse events of antibody–drug conjugates in clinical trials: a systematic review and meta‐analysis. Cancer. 2023;129(2):283–95.
Nguyen TD, Bordeau BM, Balthasar JP. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers. 2023;15(3):713.
Mahalingaiah PK, et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol Ther. 2019;200:110–25.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China, China (Grant No.: 82204758 and 82073402).
Author information
Authors and Affiliations
Contributions
Z.W. Fu and C. Shi contributed to the conceptualization of the study, development of methodology, and data analysis. Z.W. Fu and C. Shi provided funding and support for this study. Z.W. Fu, C. Gao, J.Y. **e, S.J. Li, and C. Shi performed the database searches and reference review. Z.W. Fu, C. Gao, S.J. Li, and C. Zhang contributed to the assessment of study quality and data interpretation. Z.W. Fu, C. Gao, M. Gu, and C. Shi wrote the original draft of the manuscript. M. Gu, and C. Shi helped to review and write the final report. All authors have read and approved the submission of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fu, Z., Gao, C., **e, J. et al. Incidence and risk of fatal adverse events in cancer patients treated with HER2-targeted antibody-drug conjugates: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 23, 960 (2023). https://doi.org/10.1186/s12885-023-11250-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11250-1