Abstract
Background
PTPRF interacting protein alpha 1 (PPFIA1) is reportedly related to the occurrence and progression of several kinds of malignancies. However, its role in esophageal squamous cell carcinoma (ESCC) is unclear. This current study investigated the prognostic significance and biological functions of PPFIA1 in ESCC.
Methods
Oncomine, Gene Expression Profiling Interactive Analysis (GEPIA), and Gene Expression Omnibus (GEO) were used to investigate PPFIA1 expression in esophageal cancer. The relationship between PPFIA1 expression and clinicopathological characteristics and patient survival was evaluated in GSE53625 dataset, and verified in the cDNA array based on qRT–PCR and tissue microarray (TMA) dataset based on immunohistochemistry. The impact of PPFIA1 on the migration and invasion of cancer cells were investigated by wound-healing and transwell assays, respectively.
Results
The expression of PPFIA1 was obviously increased in ESCC tissues versus adjacent esophageal tissues according to online database analyses (all P < 0.05). High PPFIA1 expression was closely related to several clinicopathological characteristics, including tumor location, histological grade, tumor invasion depth, lymph node metastasis, and tumor-node-metastasis (TNM) stage. High PPFIA1 expression was related to worse outcomes and was identified as an independent prognostic factor of overall survival in ESCC patients (GSE53625 dataset, P = 0.019; cDNA array dataset, P < 0.001; TMA dataset, P = 0.039). Downregulation of PPFIA1 expression can significantly reduce the migration and invasion ability of ESCC cells.
Conclusion
PPFIA1 is related to the migration and invasion of ESCC cells, and can be used as a potential biomarker to evaluate the prognosis of ESCC patients.
Similar content being viewed by others
Background
Esophageal cancer is ranked as the eighth most common cancer and the sixth leading cause of cancer-related death worldwide [1]. Esophageal squamous cell carcinoma (ESCC) is the main pathological subtype, accounting for nearly 90% of esophageal cancer tumors in China [2]. Surgical resection and neoadjuvant or adjuvant therapy are the main methods for ESCC treatment. Despite advances in diagnosis and therapy, recurrence and metastasis are common and significantly decrease the overall survival (OS) of patients [Statistical analyses Statistical analyses were performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL) and GraphPad Prism 7.0 software (GraphPad, Inc., La Jolla, CA, USA). Data were expressed as the mean ± standard deviation (SD). The differences between the groups were assessed using Student's t test. The patients were divided into two groups according to the best cutoff value of PPFIA1 expression for predicting OS calculated with the X-tile 3.6.1 software (Yale University, New Haven, CT, USA) for analysis of the GSE53625 dataset [18] or according to the median value of PPFIA1 expression for analysis of the cDNA array dataset. The chi-square test or Fisher’s exact test was used for the analysis of PPFIA1 expression and clinicopathological variables. Survival analysis was carried out through the Kaplan–Meier method and the log-rank test. The correlations between PPFIA1 expression and the expression of other genes were assessed by Pearson’s correlation analysis. A P value < 0.05 was deemed statistically significant.
Results
The expression of PPFIA1 mRNA in various tumor tissues
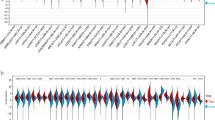
We first examined the expression level of PPFIA1 mRNA in various tumors with the GEPIA website, and the results showed significantly higher PPFIA1 mRNA expression in several types of tumor tissues, including esophageal cancer (ESCA), pancreatic adenocarcinoma (PAAD), and thymoma tissues, compared with adjacent normal tissues (Fig. 1A). Overall PPFIA1 expression in various tumor specimens, as well as in normal controls, were further analyzed with the Oncomine database. PPFIA1 mRNA expression was obviously increased in the tumor tissues versus normal tissues from most of the datasets for all types of tumors (Fig. 1B). The fold changes in PPFIA1 mRNA expression in different ESCA tissues of 7 datasets are displayed in Supplementary Table 1.
The expression of PPFIA1 was upregulated in ESCC tissues
The expression of PPFIA1 mRNA was markedly higher in ESCA tumor samples than in adjacent normal samples according to the GEPIA website (Fig. 2A). This finding was further verified using data from six GEO datasets; the results indicated that the relative PPFIA1 mRNA expression was obviously upregulated in ESCC tissues compared with paired or unpaired adjacent normal tissues in the GSE23400 (9.6 ± 1.1 vs. 8.4 ± 0.6, P < 0.001, Fig. 2B), GSE20347 (10.3 ± 1.3 vs. 8.4 ± 0.2, P < 0.001, Fig. 2C), GSE29001 (6395.6 ± 4748.9 vs. 1498.6 ± 337.9, P = 0.005, Fig. 2D), GSE53625 (12.6 ± 1.2 vs. 11.0 ± 0.5, P < 0.001, Fig. 2E), GSE45670 (251.5 ± 185.9 vs. 97.5 ± 49.6, P = 0.015, Fig. 2F) and GSE26886 (1.3 ± 1.4 vs. 0.1 ± 1.1, P = 0.022, Fig. 2G) datasets.
The expression of PPFIA1 mRNA in ESCC patients. A PPFIA1 mRNA expression in ESCC tissues versus normal tissues was analyzed using GEPIA datasets. PPFIA1 mRNA was highly expressed in ESCC tissues compared to noncancerous tissues in the GSE23400 (B), GSE20347 (C), GSE29001 (D), GSE53625 (E), GSE45670 (F) and GSE26886 (G) datasets. H PPFIA1 mRNA expression was determined in 67 tumor tissues and 28 normal tissues (cDNA array) using qRT–PCR analysis. *P < 0.05 comparison between normal and tumor tissues
In addition, the increased expression of PPFIA1 mRNA in ESCC samples was further confirmed through the analysis of cDNA array data based on qRT–PCR (P = 0.003, Fig. 2H).
Correlations between PPFIA1 mRNA expression and clinicopathological variables in ESCC
In the GSE53625 dataset, the 179 ESCC patients were divided into a low-expression group (n = 155) and a high-expression group (n = 24) according to the X-tile cutoff. Our results showed that high PPFIA1 expression was positively related to tumor invasion depth (P = 0.019), lymph node metastasis (P = 0.013), and TNM stage (P = 0.012), but no correlations were found between PPFIA1 expression and gender, age, smoking use, alcohol use, tumor location, histological grade or adjuvant therapy (P > 0.05) (Table 1).
We then analyzed PPFIA1 levels in the cDNA array data of 67 ESCC patients. The patients were grouped into a low-expression group (n = 34) and a high-expression group (n = 33) based on the median value of relative PPFIA1 expression. We found that PPFIA1 mRNA expression was significantly correlated with histological grade (P = 0.031, Supplementary Table 2).
Correlations between PPFIA1 protein expression and clinicopathological variables in ESCC patients in the TMA dataset
We further assessed PPFIA1 protein expression using an IHC staining-based TMA dataset containing samples from 147 surgically removed cancer tissues and 40 normal esophageal tissues. PPFIA1 was primarily localized to the cytoplasm and nucleus of cancer cells, and the expression rate was significantly higher in cancer tissues than in normal tissues (68.0% vs. 25.0%, P < 0.05). Representative images of low or high PPFIA1 expression are shown in Fig. 3A-C. All patients were divided into the PPFIA1 low-expressing group (n = 47) or the high-expressing group (n = 100). Correlation analysis found that PPFIA1 expression was inversely associated with tumor location (P = 0.011), tumor invasion depth (P = 0.041), lymph node metastasis (P = 0.020), and TNM stage (P = 0.007) in ESCC patients (Table 2).
Increased PPFIA1 expression indicates a poor prognosis for malignancies
In view of the high expression of PPFIA1 mRNA in a variety of malignancies, including breast, ovarian, lung, and gastric cancers, we further investigated the prognostic value of PPFIA1 through the Kaplan–Meier Plotter website. Patients were grouped based on the automatically selected best cutoff value for PPFIA1 expression. As shown in Supplementary Fig. 1, the expression of PPFIA1 apparently correlated with the RFS, OS, PPS, and DMFS of patients with breast cancer (P < 0.05); the PFS, OS, and PPS of patients with ovarian cancer (P < 0.05); the FP, OS, and PPS of patients with lung cancer (P < 0.05); and the FP, OS, and PPS of patients with gastric cancer (P < 0.05). These results suggest that high expression of PPFIA1 is obviously associated with worse outcomes in patients with breast cancer, ovarian cancer, lung cancer, and gastric cancer.
Correlations between PPFIA1 expression and the prognosis of ESCC patients
Survival analyses revealed that the median survival time of ESCC patients with high and low PPFIA1 expression were similar (22.7 months vs. 45.37 months, P = 0.349, Fig. 4A) in the TCGA database. ESCC patients with high PPFIA1 mRNA levels had a significantly worse 5-year OS rate than those with low PPFIA1 mRNA levels in the GSE53625 dataset (12.5% vs. 45.2%, P < 0.001, Fig. 4B) and cDNA array dataset (9.2% vs. 38.0%, P = 0.001, Fig. 4C). High PPFIA1 expression at the protein level was also obviously related to poor outcomes in patients in the TMA dataset (5-year OS rate: 23.4% vs. 46.0%, P = 0.002, Fig. 4D).
The association of PPFIA1 expression with the OS of ESCC patients. A Kaplan‒Meier curve of the survival of patients stratified by PPFIA1 mRNA expression in the TCGA dataset. B Kaplan‒Meier curve of the survival of patients stratified by PPFIA1 mRNA expression in the GSE53625 dataset. C Kaplan‒Meier curve of the survival of patients stratified by PPFIA1 mRNA expression in the cDNA array dataset. D Kaplan‒Meier curve of PPFIA1 protein expression stratification in the TMA dataset
The prognosis-related indicators in the 3 datasets were analyzed by univariate and multivariate survival analyses. In the GSE53625 dataset, age, histological grade, lymph node metastasis, adjuvant therapy and PPFIA1 expression were significantly related to OS. In the cDNA array dataset, lymph node metastasis, M status, TNM stage and PPFIA1 expression were related to OS. Moreover, age, tumor size, histological grade, tumor invasion depth, lymph node metastasis, and PPFIA1 expression were significantly related to OS in the TMA dataset. Furthermore, PPFIA1 was identified as an independent risk factor for poor OS in the multivariate Cox proportional hazard regression analysis of all 3 datasets (GSE53625 dataset, HR = 1.942, P = 0.019, Fig. 5A; cDNA array dataset, HR = 3.464, P < 0.001, Fig. 5B; TMA dataset, HR = 1.596, P = 0.039, Fig. 5C). Other independent factors included tumor size (TMA dataset), lymph node metastasis (GSE53625 and TMA datasets), TNM stage (cDNA array dataset) and adjuvant therapy (GSE53625 dataset).
The effect of PPFIA1 on the migration and invasion of ESCC cells in vitro
To further determine the role of PPFIA1 in ESCC cell migration and invasion, we transfected Kyse-30 and Ec-109 cells with PPFIA1-siRNA or negative control. We found the PPFIA1 expression at the mRNA (Fig. 6A) and protein (Fig. 6B) levels were downregulated by transfection with PPFIA1-siRNA (P < 0.05). The wound-healing assay showed that PPFIA1 knockdown significantly inhibited the migration ability of Kyse-30 and Ec-109 cells (Fig. 6C, P < 0.05). Furthermore, transwell assays showed that the relative migration and invasion abilities of Kyse-30 and Ec-109 cells were remarkably decreased after PPFIA1 knockdown (Fig. 6D, P < 0.05). Thus, these results indicate that the expression of PPFIA1 is crucial for the migration and invasion of ESCC cells.
Effects of PPFIA1 knockdown on the migration and invasion of ESCC cells in vitro. The expression of PPFIA1 in Kyse-30 and Ec-109 cells after transfection with PPFIA1-siRNA was determined by qRT‒PCR (A) and western blotting (B), respectively. C Cell migration of ESCC cells was assessed by wound-healing assay. D Cell migration and invasion of ESCC cells were assessed by transwell assays. *P < 0.05
Network analysis of PPFIA1 and interacting genes
The interactions between PPFIA1 and other genes in the PPI network were analyzed via the STRING website. The results showed some known and predicted interactions between PPFIA1 and UNC13B, RAB3A, PTPRD, SYT1, RIMS1, PTPRS, APBA1, PTPRF, LIN7A, and CASK (Fig. 7A). Most of these genes have been reported as oncogenes or tumor suppressor genes.
PPI network analysis of PPFIA1-related proteins. A Annotation of the genes that were associated with PPFIA1 using the STRING database. The correlations of PPFIA1 expression with UNC13B (B), RAB3A (C), PTPRD (D), SYT1 (E), RIMS1 (F), PTPRS (G), APBA1 (H), PTPRF (I), LIN7A (J), and CASK (K) expression were assessed by Pearson’s correlation analysis in the GSE53625 dataset
We further analyzed the associations of PPFIA1 mRNA expression with the abovementioned genes in the GSE53625 dataset. Our results showed that PPFIA1 expression was clearly correlated with UNC13B, RAB3A, PTPRD, SYT1, RIMS1, PTPRS, APBA1, PTPRF, and LIN7A expression (Fig. 7B-J) but not with CASK expression (Fig. 7K).
Discussion
Early invasion and metastasis are two of the main reasons for the poor prognosis of patients with ESCC [19]. In our current study, we observed an increase of PPFIA1 expression in ESCC, which was associated with tumor metastasis and a poorer prognosis. PPFIA1 may be a potential molecular indicator for diagnosis and prognosis evaluation and could be regarded as a new therapeutic target for ESCC.
PPFIA1 is a gene located at the 11q13 amplification region and mainly encodes the liprin-α1 protein in humans. It was initially discovered to control the formation and function of synapses in neurons [20]. Studies have indicated that PPFIA1 is a key regulator of cell motility, focal adhesion, cell signal transduction, and cytoskeletal organization [21, 22]. PPFIA1 is frequently amplified and plays a crucial role in cell migration and invasion by affecting cell motility, mediating extracellular matrix degradation and facilitating the formation of lamellipodial protrusions of cancer cells [5, 23, 24]. Although the role of PPFIA1 in tumor cell progression has been well verified, it is not clear whether its dysregulation is related to metastasis risk or prognosis in ESCC patients.
To determine the clinical value of PPFIA1 in ESCC, we analyzed the difference in the expression of this novel marker in tumor tissues compared with normal tissues and assessed its correlation with the survival of ESCC patients using multiple datasets. Analysis of datasets from Oncomine, GEPIA and GEO confirmed that PPFIA1 mRNA expression was markedly higher in ESCC tissues than in adjacent normal control tissues, which was further confirmed with cDNA array data based on qRT–PCR and TMA data based on IHC. These results indicated that PPFIA1 may play a vital role during the tumorigenesis of ESCC, which is consistent with the results discovered in other malignant tumors [20, 23]. Further correlation analysis revealed that PPFIA1 expression is highly correlated with aggressive biological behaviors of tumors, indicating the important function of PPFIA1 in the progression of ESCC. It is worth mentioning that the expression of PPFIA1 was only correlated with the degree of tumor differentiation in the cDNA chip dataset, which might be due to the limited sample size in the research.
We further discovered that PPFIA1 can be applied for the prognostic assessment of breast cancer, ovarian cancer, lung cancer and gastric cancer by online Kaplan–Meier Plotter. Cho et al. [25]. explored the prognostic value of PPFIA1 alone or in combination with TMEM16A and FADD in patients with invasive breast cancer and found that combined expression was significantly associated with perineural invasion and a low disease-free survival rate. A recent study showed that the expression of PPFIA1 is closely related to poor response to endocrine treatment in luminal breast cancer [21]. Since PPFIA1 was found to be involved in the development of ESCC, it could be inferred that PPFIA1 is likely to have a great impact on the survival of patients with ESCC. To assess this possibility, we used the GSE53625, cDNA array and TMA datasets to investigate the prognostic value of PPFIA1 in ESCC. Our results indicated that high PPFIA1 expression was evidently correlated with a poorer prognosis. Notably, PPFIA1 was identified as an independent indicator of poor prognosis in all three independent databases in the multivariate analyses. Thus, PPFIA1 may serve as a potential diagnostic and novel prognostic biomarker, as well as a new therapeutic target for ESCC.
Since the expression of PPFIA1 is related to malignant biological indicators and poor prognosis, we further carried out cytological experiments to clarify the effect of PPFIA1 on the metastasis ability of ESCC cells. Our study found that down-regulation of PPFIA1 expression can significantly reduce the migration and invasion behavior of cancer cells. These data further support that PPFIA1 functions as an oncogene in the metastasis of ESCC.
Mechanistically, Shen et al. [26] confirmed that PPFIA1 enhances the proliferation and migration ability of colon carcinoma cells by interacting with the tumor suppressor protein ING4. Astro et al. [27] suggested that liprin-α1 can determine the polarization and morphological dynamics related to cell migration by forming a complex with the liprin-β1, ERC1/ELKS, and LL5 proteins. To examine the potential mechanisms of PPFIA1 in ESCC progression, we further used PPI network and Pearson’s correlation analyses to identify proteins that may bind to PPFIA1. We identified UNC13B, RAB3A, PTPRD, SYT1 and RIMS1 as potential candidates. Some of these interacting genes have already been reported as tumor oncogenes or suppressor genes. For instance, RAB3A interacting protein (Rab3IP) is a Rab-specific GEF, and the activation of Rab proteins, including RAB3A and RAB8, has been considered a tumor-specific marker in colorectal cancer, gastric cancer, and pancreatic cancer [28, 29]. A recent study reported by Ren et al. [30] showed that RAB3IP interacts with SSX2 and enhances the invasive aggressive phenotype of gastric cancer through epithelial-mesenchymal transition. PTPRT is a phosphatase that can participate in JAK/STAT signal transduction. Deleterious mutations or copy number loss of PTPRT and its related gene PTPRD are potential markers for evaluating resistance to bevacizumab regimens and are closely associated with shorter PFS in metastatic colorectal cancer patients [31]. However, the biological function and underlying mechanism of PPFIA1 in ESCC need to be further studied.
There are several certain limitations. First, although the results were analyzed through bioinformatics analysis and three independent databases (GSE53625, cDNA array and TMA), the sample size involved in the present study was relatively small. Second, the clinicopathological information from the three datasets was not comprehensive, and part of the information in the cDNA array dataset was incomplete. Third, the detailed molecular mechanism and signaling pathways by which PPFIA1 influences the migration and invasion of ESCC were not verified. Therefore, our results need to be further verified.
In conclusion, this study indicates that the expression of PPFIA1 is significantly increased and is related to migration and invasion behaviors, and poor outcomes in ESCC patients. PPFIA1 might be a valuable biomarker for early detection, treatment formulation and prognostic evaluation for ESCC.
Availability of data and materials
The datasets that support the findings of this study are available from third parties, with the weblinks provided in the manuscript methodology section and can be located at the following sites: GEPIA (http://gepia.cancer-pku.cn/detail.php), Oncomine (http://www.oncomine.org), GEO database (https://www.ncbi.nlm.nih.gov/geo/), GSE53625 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE53625). TCGA database (https://cancergenome.nih.gov/), Kaplan–Meier Plotter (www.kmplot.com) and STRING (http://string-db.org/).
Abbreviations
- ESCC:
-
Esophageal squamous cell carcinoma
- PPFIA1:
-
PTPRF interacting protein alpha 1
- GEPIA:
-
Gene Expression Profiling Interactive Analysis
- TMA:
-
Tissue microarray
- PPI:
-
Protein–protein interaction
- STRING:
-
Search Tool for the Retrieval of Interacting Genes
- RFS:
-
Recurrence-free survival
- PFS:
-
Progression-free survival
- FP:
-
Time to first progression
- OS:
-
Overall survival
- PPS:
-
Post-progression survival
- DMFS:
-
Distant metastasis-free survival
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Morgan E, Soerjomataram I, Rumgay H, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649-58.e2.
Zhang H, **ao W, Ren P, et al. The prognostic performance of the log odds of positive lymph nodes in patients with esophageal squamous cell carcinoma: a population study of the US SEER database and a Chinese single-institution cohort. Cancer Med. 2021;10(17):6149–64.
Pehkonen H, von Nandelstadh P, Karhemo PR, et al. Liprin-α1 is a regulator of vimentin intermediate filament network in the cancer cell adhesion machinery. Sci Rep. 2016;6:24486.
Astro V, Asperti C, Cangi MG, Doglioni C, de Curtis I. Liprin-α1 regulates breast cancer cell invasion by affecting cell motility, invadopodia and extracellular matrix degradation. Oncogene. 2011;30(15):1841–9.
Blessmann M, Al-Dam A, Hanken H, et al. Amplification of the PPFIA1 gene region on 11q13 in oral squamous cell carcinomas (OSCC). J Craniomaxillofac Surg. 2013;41(8):845–9.
Brown LA, Kalloger SE, Miller MA, et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer. 2008;47(6):481–9.
Chu J, Min KW, Kim DH, et al. High PPFIA1 expression promotes cancer survival by suppressing CD8+ T cells in breast cancer: drug discovery and machine learning approach. Breast Cancer. 2023;30(2):259–70.
Fu F, Niu R, Zheng M, et al. Clinicopathological significances and prognostic value of PPFIA4 in colorectal cancer. J Cancer. 2023;14(1):24–34.
Zhou H, Cao T, Li WP, Wu G. Combined expression and prognostic significance of PPFIA1 and ALG3 in head and neck squamous cell carcinoma. Mol Biol Rep. 2019;46(3):2693–701.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98-98W102.
Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9(2):166–80.
Yu-**g T, Wen-**g T, Biao T. Integrated analysis of hub genes and pathways in esophageal carcinoma based on NCBI’s Gene Expression Omnibus (GEO) database: a bioinformatics analysis. Med Sci Monit. 2020;26:e923934.
**ao W, Tang P, Sui Z, et al. Establishment of a risk model by integrating hypoxia genes in predicting prognosis of esophageal squamous cell carcinoma. Cancer Med. 2023;12(2):2117–33.
Alatan H, Chen Y, Zhou J, Wang L. Extracellular matrix-related hubs genes have adverse effects on gastric adenocarcinoma prognosis based on bioinformatics analysis. Genes (Basel). 2021;12(7):1104.
Zhang H, Wu X, Sui Z, et al. High-mobility group AT-hook 2 promotes growth and metastasis and is regulated by miR-204-5p in oesophageal squamous cell carcinoma. Eur J Clin Invest. 2021;51(8):e13563.
Wu X, Zhang H, Sui Z, et al. CXCR4 promotes the growth and metastasis of esophageal squamous cell carcinoma as a critical downstream mediator of HIF-1α. Cancer Sci. 2022;113(3):926–39.
Ogłuszka M, Orzechowska M, Jędroszka D, Witas P, Bednarek AK. Evaluate cutpoints: adaptable continuous data distribution system for determining survival in Kaplan-Meier estimator. Comput Methods Programs Biomed. 2019;177:133–9.
Zhang J, Luo A, Huang F, Gong T, Liu Z. SERPINE2 promotes esophageal squamous cell carcinoma metastasis by activating BMP4. Cancer Lett. 2020;469:390–8.
Barros-Filho MC, Reis-Rosa LA, Hatakeyama M, et al. Oncogenic drivers in 11q13 associated with prognosis and response to therapy in advanced oropharyngeal carcinomas. Oral Oncol. 2018;83:81–90.
Alfarsi LH, El Ansari R, Craze ML, et al. PPFIA1 expression associates with poor response to endocrine treatment in luminal breast cancer. BMC Cancer. 2020;20(1):425.
Dancau AM, Wuth L, Waschow M, et al. PPFIA1 and CCND1 are frequently coamplified in breast cancer. Genes Chromosomes Cancer. 2010;49(1):1–8.
Chiaretti S, de Curtis I. Role of Liprins in the regulation of tumor cell motility and invasion. Curr Cancer Drug Targets. 2016;16(3):238–48.
Yang J, Wu NN, Huang DJ, et al. PPFIA1 is upregulated in liver metastasis of breast cancer and is a potential poor prognostic indicator of metastatic relapse. Tumour Biol. 2017;39(7):1010428317713492.
Choi EJ, Yun JA, Jabeen S, et al. Prognostic significance of TMEM16A, PPFIA1, and FADD expression in invasive ductal carcinoma of the breast. World J Surg Oncol. 2014;12:137.
Shen JC, Unoki M, Ythier D, et al. Inhibitor of growth 4 suppresses cell spreading and cell migration by interacting with a novel binding partner, liprin alpha1. Cancer Res. 2007;67(6):2552–8.
Astro V, Chiaretti S, Magistrati E, Fivaz M, de Curtis I. Liprin-α1, ERC1 and LL5 define polarized and dynamic structures that are implicated in cell migration. J Cell Sci. 2014;127(Pt 17):3862–76.
Guo W, Chen Z, Chen Z, et al. Promotion of Cell Proliferation through inhibition of cell autophagy signalling pathway by Rab3IP is restrained by MicroRNA-532-3p in gastric cancer. J Cancer. 2018;9(23):4363–73.
Hur K, Cejas P, Feliu J, et al. Hypomethylation of long interspersed nuclear element-1 (LINE-1) leads to activation of proto-oncogenes in human colorectal cancer metastasis. Gut. 2014;63(4):635–46.
Ren H, Xu Z, Guo W, Deng Z, Yu X. Rab3IP interacts with SSX2 and enhances the invasiveness of gastric cancer cells. Biochem Biophys Res Commun. 2018;503(4):2563–8.
Hsu HC, Lapke N, Chen SJ, et al. PTPRT and PTPRD deleterious mutations and deletion predict bevacizumab resistance in metastatic colorectal cancer patients. Cancers (Basel). 2018;10(9):314.
Acknowledgements
Not Applicable.
Funding
This work was supported by the National Natural Science Foundation of China (82002551, 81772619), Science and Technology Project of Tian** Municipal Health Commission (KJ20119), Bethune Charitable Foundation-Excelsior Surgical Fund (CESS2021TB01, HZB-20190528–18), and Clinical Trial Project of Tian** Medical University (2017kylc006).
Author information
Authors and Affiliations
Contributions
YY.G., LZ.G., P.T., ZT.Y. and HD.Z. conceived and designed the workflow. R.J., WY.X., YM.H. and Y.L. performed the experiments and analyzed the data. R.J. and WY.X. conducted the bioinformatics analysis. YY.G. and YM.H. contributed to the histological analysis. Y.L. contributed to the bioinformatics analysis. YY.G. and LZ.G. wrote the manuscript. P.T., ZT.Y. and HD.Z. participated in the revision of the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant ethical regulations and were conducted under approvals of the Research Ethics Committee of Tian** Medical University Cancer Institute and Hospital. Informed consent was acquired from all patients of primary cohort for the acquisition of clinical and pathological information and the use of surgical specimens.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Oncomine analysis of PPFIA1 mRNA expression in esophageal cancer.
Additional file 2:
Supplementary Table 2. Correlations between PPFIA1 mRNA expression and clinicopathological variables in 67 ESCC patients in the cDNA array dataset.
Additional file 3:
Supplementary Fig. 1. The correlations between PPFIA1 expression and the prognoses of patients with malignancies were analyzed using Kaplan-Meier Plotter.
Additional file 4:
Supplementary Fig. 2. The full-length images of western blots in Fig. 6B.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, Y., Guan, L., Jia, R. et al. High expression of PPFIA1 in human esophageal squamous cell carcinoma correlates with tumor metastasis and poor prognosis. BMC Cancer 23, 417 (2023). https://doi.org/10.1186/s12885-023-10872-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10872-9