Abstract
Background
Stroke survivors have long-term upper limb impairment, which impacts the quality of life (QOL) and social reintegration, but there is lack of effective therapeutic strategies and novel technologies. Customized multi-muscle functional electrical stimulation (FES) based on the muscle synergy of healthy adults and robotic-assisted therapy (RAT) have been proved efficacy respectively. Synergy-based FES combined with RAT can be a novel and more effective therapy for upper limb recovery of stroke survivors from the perspective of synergistic enhancement. However, few studies have examined the effectiveness of combined synergy-based FES and RAT, especially for motor control evaluated by reach-to-grasp (RTG) movements. The main objective of the following research protocol is to evaluate the effectiveness and efficacy, as well as adoptability, of FES-RAT and FES or RAT rehabilitation program for upper limb function improvement after stroke.
Methods
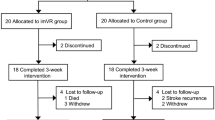
This will be an assessor-blinded randomized controlled trial involving a 12-week intervention and a 6-month follow-up. Stratified randomization will be used to equally and randomly assign 162 stroke patients into the FES + conventional rehabilitation program (CRP) group, RAT + CRP group and FES-RAT + CRP group. Interventions will be provided in 5 sessions per week, with a total of 60 sessions. The primary outcome measurements will include the Fugl-Meyer Assessment and Biomechanical Assessment of RTG movements. The secondary outcome measurements will include quality of life and brain neuroplasticity assessments by MRI. Evaluations will be performed at five time points, including at baseline, 6 weeks and 12 weeks from the start of treatment, and 3 months and 6 months following the end of treatment. A two-way analysis of variance with repeated measures will be applied to examine the main effects of the group, the time factor and group-time interaction effects.
Discussion
The results of the study protocol will provide high quality evidence for integrated synergy-based FES and RAT, and synergy-based FES alone and guide the design of more effective treatment methods for stroke rehabilitation.
Trial registration
ChiCTR2300071588.
Similar content being viewed by others
Background
Stroke is the leading cause of long-term disability among middle-aged and elderly adults worldwide [1, 2]. Approximately 70–80% of stroke survivors have limb motor impairments, especially upper limb impairment, which directly impacts the quality of life (QOL) and social reintegration [3]. There is an important effort worldwide to establish therapeutic strategies and novel technologies to improve upper limb function after stroke, such as functional electrical stimulation (FES) [4], robotic-assisted therapy (RAT) [5], and task-oriented therapy (TOT) [6]. Unfortunately, six months after stroke, approximately 65% of patients still cannot incorporate the affected arm and hand into their daily activities [7, 8]. Therefore, the primary goal in the clinical field of upper limb rehabilitation for stroke patients is the continuous exploration of novel and effective treatment therapies.
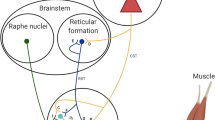
The central nervous system (CNS) recruits a reduced and fixed set of coordinated patterns of muscle activities to generate a vast variety of movements, which is defined as muscle synergy [9, 10], and several neuroscience studies have demonstrated that muscle synergy is the neurological mechanism of motor execution and control [Functional electrical stimulation (FES) A synergy-based multi-channel FES device was developed using the Fourier ElectroFortis Programmable Stimulator, which can stimulate multiple muscles with customized parameters. An experimenter computer GUI application was created to configure the following basic stimulation parameters: the frequency, minimum and maximum intensity, pulse duration, ramp time, synchronization and order of stimulations, type of user interactions and number of repetitions [30]. The stimulation envelope will be specified as the concatenated piecewise linear functions (e.g., rising phase, plateau phase and falling phase), and will be allowed to be preloaded to the FES device [30]. As participants improve, stimulation will gradually be reduced to a minimum and eventually phased out. Before the intervention, all operators will receive the homogenization training of the FES treatment scheme. The specific steps are as follows: Identify the functions to be trained and select the order of the tasks to be retrained. Identify the stimulated muscle and apply self-adhesive electrodes over the identified motor muscles: including the biceps brachii, triceps brachii lateral head, triceps brachii long head, anterior deltoid, posterior deltoid, pectoralis major, and brachioradialis [33] (Fig. 2). Identify and record the different stimulation thresholds: • the motor threshold: when a palpable or a visible contraction is produced; • the sensory threshold: when the participants feel the current for the first time; and • the maximum threshold: beyond which the patient does not tolerate an increase in current amplitude. Preloaded the muscle synergy pattern of the healthy controls to the FES device. Explain what to expect when FES is started and instruct the participants to make an active attempt to perform the intended movement. Repeat the stimulus protocol and ensure an adequate rest time. Turn off the stimulator when the treatment is completed, remove the electrodes and inspect the skin underneath for any redness. The Fourier intelligence upper-limb robotic system (Fourier, Shanghai, China), which is an intelligent rehabilitation training system designed especially for stroke patients with upper limb or motor dysfunction, will be used for robotic-assisted therapy in this study. The system can provide passive, assistive, and active planar movements around the shoulder, elbow, and wrist joints [31]. More importantly, the system is able to establish the movement trajectory of the manipulator according to the upper limb movement pattern during ADLs or target tasks, which can be customized for specific tasks. The largest body of clinical evidence has been amassed for this robotic system; the system has been successfully tested on over 500 stroke patients in clinical studies, and there are approximately 100 robots in use worldwide [34]. During treatment, participants will perform the RTG task displayed in a game scene that generates real-time auditory and visual feedback [35] (Fig. 3). Each participant will be required to sit on a chair, and his or her affected UE will be strapped to the robot arm. Machine settings such as the height of the platform, the length of the robot arm, and the amount of assistance force will be adjusted according to each participant’s personal characteristics. The treatment content (game tasks/motor or task-oriented training) settings and doses will be the same for all participants in the middle activity range. RAT will provide repetitive and high-intensity training in a cost-effective manner. When participants perform the RTG movement, the objects will be exhibited on the computer screen using a virtual hand, allowing them to receive timely feedback on their performance from the robotic device. The robot’s UE movement patterns are functional and the joints are isolated, which minimizes synergic movement in stroke patients. For FES-RAT treatment, the participants will receive FES when performing the RAT task (Fig. 4). The frequency, repeated time and rest time will be consistent with separate FES or RAT. To realize the synchronous triggering and control of FES and RAT, the control terminal will be developed through a multifunctional I/O Device (NI6255, National Instruments Inc., Austin, TX) [36]. The FES stimulus envelope and manipulator kinematic datasets will be aligned via the start switch trigger recorded digitally in HANDoVR, and the analog reading of the digital output will be sent from HANDoVR to MATLAB. To improve treatment adherence and reduce the likelihood of participants drop** out of the study, the therapists will contact the participants by texting, voice and video through an intelligent follow-up system, which is a small program embedded in WeChat app. The therapists will also regularly phone the participants if they have difficult in receiving information on smartphones. Through above strategies, the research team can confirm their appointments, discuss the subsequent intervention step as well as avoiding any barriers to adherence. All outcome measurements will be assessed by a professional rehabilitation assessor who will be blinded at baseline (T1), 6 weeks (T2) and 12 weeks (T3) during the intervention and at 3 months (T4) and 6 months (T5) during the follow-up period. Data on the demographic and clinical characteristics of the participants will be collected at T1. The primary outcome is upper extremity function evaluated by the upper extremity section of the Fugl-Meyer scale. The FMA-UE has outstanding intrarater (ICC = 0.997) and interrater (ICC = 0.993) reliability for assessing proximal-to-distal and synergistic-to-isolated movement behavior in stroke patients [37, 38]. The highest possible total score is 66, with 33 items and ordinal scoring from 0 to 2, with a higher score indicating more effective function. Additionally, both the proximal (range 0–42) and distal (range 0–24) subscores of the FMA-UE will also be considered. The RTG movements of the affected upper extremity will be assessed by three-dimensional biomechanical analysis. The RTG performance test will be selected because prior research has demonstrated that concentrating purely on task outcome measures fails to differentiate between neural recovery processes and the development of efficient but abnormal compensatory movement patterns [39,40,41,42]. Multiple authors have utilized the kinematic analysis of three-dimensional RTG movements as a viable means to analyze the motor control process and identify the normalization of motor function in persons with stroke [40, 42, 43]. The biomechanical evaluation protocol will be based on a previously established, standardized kinematic analysis testing protocol for RTG movements (i.e., drinking) [40, 42]. More comprehensively, the present study protocol will further complement the evaluation of muscle activation and synergy patterns. The standardization of the RTG task will be divided into five phases: (1) reaching to grasp a glass, (2) forward transport of the glass to the mouth, (3) drinking a sip of water, (4) transporting the glass back to the table, and (5) returning the hand to the initial position [41]. The details of the test task are as follows: the participants will sit comfortably at a table, with 90° knee and hip flexion and 90° elbow flexion with the upper arm vertical and forearm horizontal. The gras** target will be rigidly mounted on the testing table in front of the subject, aligned with the participant’s affected shoulder and at a distance of 35 cm. Data from each participant will be collected from 5 successful trials, and the rest time between each trial will be approximately 30 s. Before the biomechanical test, the reflected markers and EMG sensors will be placed on the anatomical landmarks and targeted muscle respectively. Eight markers will be affixed on the tested hand (III metacarpophalangeal joint), the wrist (styloid process of the ulna), the elbow (lateral epicondyle), on both shoulders (acromion), the trunk (sternum), the forehead and the drinking glass. EMG sensors will be placed on the following ten muscles of each participant’s shoulder, arm and hand on the affected side: the first dorsal interosseous (FDI), flexor digitorum superficialis (FDS), extensor digitorum communis (EDC), extensor indicis (EI), abductor pollicis brevis (APB), extensor pollicis brevis (EPB), biceps brachii (BB), triceps brachii (TB), anterior deltoid (AD), and posterior deltoid (PD). A 10–camera infrared motion capture system (sampling rate: 100 Hz; Vicon Motion Systems, Oxford, United Kingdom) and a Delsys EMG system (sampling rate: 1000 Hz; Delsys Inc., Natick, MA) will acquire three-dimensional kinematics and muscle activation information, respectively. The outcomes of kinematic analysis will include the following: the movement time (ms), peak velocity (cm/s), time to peak transport velocity (ms), trajectory length ratio (%), trajectory smoothness, peak aperture (cm), peak aperture velocity (cm/s), opening distance (cm) and reach grasp coupling index. The definitions of kinematic variables have been described in several previous studies [36, 40, 42,43,44]. Additionally, the muscle synergy pattern will be extracted from the raw EMG signals through the method of non-negative matrix [11, 32], which considers muscle activation as the linear combination of multiple muscle components (muscle vectors) with the corresponding activation coefficients (time profiles). The QOL of the participants will be measured by the Stroke-Specific Quality of Life Scale (SS-QOL). The SS-QOL was created in 1999 to quantify the impacts of stroke and assessed the efficacy of treatment strategies with high reliability (0.98) [39, 40]. The SS-QOL is a long 49-item stroke-specific instrument that provides a total QOL score and 12 subscale scores associated with mobility, cognition, mood, functionality, and social roles, with higher scores indicating a better QOL. Magnetic resonance imaging (MRI) will be used to investigate the brain mechanisms of rehabilitation, particularly in regions related to motor learning and motor control. MRI data will be acquired using a 3.0 T magnetic resonance scanner (Siemens Magnetom Verio Syngo MR B17, Germany) with a 32-channel phase-array head coil at the Department of Medical Imaging of the Seventh People's Hospital of Shanghai University of Traditional Chinese Medicine. The following three MRI modalities will be acquired [41,42,43]: (1) high-resolution anatomical MRI (T1-MRI) for estimating the anatomical parameters of the cortex, the subcortex, and structural connectivity; (2) resting-state functional MRI (RS-fMRI) for estimating the functional connectivity of brain regions; and (3) diffusion tensor imaging (DTI) for estimating the tract-graphic parameters of white matter and the microstructure of gray matter. The project and data management, data analysis and data monitoring will be supervised by the independent Data Monitoring Committee (DMC) affiliated with the Scientific Research Innovation Platform of the Seventh People's Hospital of Shanghai University of Traditional Chinese Medicine. The dataset will be stored, analyzed and archived in a pseudonymized manner to entirely protect individual privacy and minimize bias. All statistical analyses will be performed with IBM SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) by statisticians who are blinded to the group allocation. The analyses will be performed on an intention-to-treat basis. The Shapiro–Wilk (SW) test will be used to examine the normal distribution of the continuous variables comprising demographic and outcome measures. Continuous variables will be described as the mean ± SD for those with normal distributions or the median for those with nonnormal distributions, and categorical variables will be described as the frequency. The χ2 test or Fisher’s exact test will be used to examine the comparisons among the three groups for categorical variables. When the normality of data distribution is determined, two-way of variance with repeated measures will be applied to examine the main effects of the group and time factors, as well as the group-time interaction effects. A simple effect post hoc analysis will be conducted when the time-group interaction is significant. Furthermore, the linear mixed model will be adjusted for age, sex and type of stroke if homogeneity is not found. The significance level for all statistical tests will be set at 0.05, corrected for multiple comparisons using the Bonferroni-adjusted method and accompanied by a 95% confidence interval. This study procedure will be conducted in accordance with the principles of the Declaration of Helsinki in its current version (for details, see www.wma.net). Ethics approval (2023-7th-HIRB-034) was granted by the Research Ethics Committee of the Ethics Committee of the Seventh People's Hospital of Shanghai University of Traditional Chinese Medicine. The results of the study will be published in peer-reviewed scientific journals and presented at conferences and workshops after study completion.
Robotic-assisted therapy (RAT)
FES-RAT
To reduce the dropout rate
Outcome measurements
Primary outcome measurements
Upper extremity function
Biomechanical assessment of reach-to-grasp movements
Secondary outcome measurements
Quality of life
Brain neuroplasticity
Data management and monitoring
Statistical analysis
Ethics and dissemination
Discussion
The recovery of motor function is an important goal in the rehabilitation of poststroke patients [7]. Customized multi-muscle functional electrical stimulation (FES) based on the muscle synergy of healthy adults may assist in improving upper limb motor function and motor control in stroke patients [12, 17, 18]. However, relevant studies have only reported the instantaneous and short-term effects of synergy-based FES. Synergy-based FES combined with robotic-assisted therapy (RAT) can be a novel and more effective therapy for upper limb function improvement of the stroke survivors, but few studies have examined its effectiveness, especially motor control in the RTG movement. Based on the above problems, the authors have designed a comprehensive research scheme framework including a 12-week intervention and a 6-month follow-up, which allows three study purposes to be realized simultaneously.
This study has several important strengths. First, it is a novel integrated treatment method for combining synergy-based FES and RAT interventions, based on integrated rehabilitation technologies and synergistic enhancement effects for improving the upper limb function after stroke. Previous studies examined individual components (e.g., CRP, FES, RAT) but have not combined them in an integrated method [4, 20, 29]. Second, the comprehensive intervention protocol will be evidence-based and rigorously developed based on the evidence, recommendations, theories and practice standards of the systematic review [4, 6]. Third, more systematic and comprehensive assessment outcomes will be selected for this protocal based on biomechanical and brain science, such as the biomechanical assessment of RTG movements (e.g., kinematics, EMG), and resting-state functional MRI (RS-fMRI) for estimating the functional connectivity of brain regions [44, 45]. Therefore, the present study will provide a more comprehensive and systematic protocol for the further study with a randomized controlled trial design in the stroke rehabilitation.
We recognize that this study also has limitations. This research has an inevitable limitation associated with the difficulty in controlling the methodology of blinding during the RAT intervention because the participants and therapists cannot be blinded due to the visibility of the RAT intervention. Although the outcome assessors and data analyzer will be blinded to the group allocation, there might still be a risk of detection bias during the study’s implementation. Additionally, muscle synergy extraction, FES and RAT training in this study need to be performed by medical personnel in medical institutions, which may limit the promotion and application of synergy-based FES and RAT in the community and home settings. Furthermore, based on the results of a few recent studies, there were no differences between intracerebral hemorrhage and cerebral infarction of function prognosis at rehabilitation discharge, so when including patients, we didn’t make a clear distinction on whether different types of strokes will affect the intervention outcomes, which should be investigated in further study.
In summary, the results of the study protocol will achieve multiple study purposes, including demonstrating the effectiveness of synergy-based FES, and synergy-based FES-RAT for improving upper limb function after stroke and exploring the efficacy differences in improving upper limb function after stroke between the FES-RAT therapy and FES or RAT. Synergy-based FES combined with RAT may have a potential opportunity to better improve the upper limb function of stroke survivors. These results of the study will provide high-quality evidence for integrated synergy-based FES and RAT and synergy-based FES alone and guide the design of more effective treatment methods for stroke rehabilitation.
Availability of data and materials
Not applicable.
Abbreviations
- FES:
-
Functional Electrical Stimulation
- RAT:
-
Robotic-assisted Therapy
- RTG:
-
Reach-To-Grasp
- CRP:
-
Conventional Rehabilitation Programs
- QOL:
-
Quality of Life
- TOT:
-
Task-oriented Therapy
- CNS:
-
Central Nervous System
- RCT:
-
Randomized Controlled Trial
- ADL:
-
Activity of Daily Living
- NMF:
-
Non-negative Matrix Factorization
- FDI:
-
First Dorsal Interosseous
- FDS:
-
Flexor Digitorum Superficialis
- EDC:
-
Extensor Digitorum Communis
- EI:
-
Extensor Indicis
- APB:
-
Abductor Pollicis Brevis
- EPB:
-
Extensor Pollicis Brevis
- BB:
-
Biceps Brachii
- TB:
-
Triceps Brachii
- AD:
-
Anterior Deltoid
- PD:
-
Posterior Deltoid
- MRI:
-
Magnetic Resonance Imaging
- RS-fMRI:
-
Resting-state Functional MRI
- DTI:
-
Diffusion Tensor Imaging
- DMC:
-
Data Monitoring Committee
- FES:
-
Functional Electrical Stimulation
References
Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019;18(5):417–8.
Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):S6–16.
Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820.
Eraifej J, Clark W, France B, Desando S, Moore D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: a systematic review and meta-analysis. Syst Rev. 2017;6(1):40.
Bressi F, Cricenti L, Campagnola B, Bravi M, Miccinilli S, Santacaterina F, Sterzi S, Straudi S, Agostini M, Paci M, et al. Effects of robotic upper limb treatment after stroke on cognitive patterns: a systematic review. NeuroRehabilitation. 2022;51(4):541–58.
da Silva ESM, Ocamoto GN, Santos-Maia GLD. de Fatima Carreira Moreira Padovez R, Trevisan C, de Noronha MA, Pereira ND, Borstad A, Russo TL: The Effect of Priming on Outcomes of Task-Oriented Training for the Upper Extremity in Chronic Stroke: A Systematic Review and Meta-analysis. Neurorehabil Neural Repair. 2020;34(6):479–504.
Knutson JS, Fu MJ, Sheffler LR, Chae J. Neuromuscular Electrical Stimulation for Motor Restoration in Hemiplegia. Phys Med Rehabil Clin N Am. 2015;26(4):729–45.
Bejot Y, Bailly H, Durier J, Giroud M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Medicale. 2016;45(12 Pt 2):e391–8.
d’Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci U S A. 2005;102(8):3076–81.
d’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6(3):300–8.
Niu CM, Bao Y, Zhuang C, Li S, Wang T, Cui L, **e Q, Lan N. Synergy-based FES for post-stroke rehabilitation of upper-limb motor functions. IEEE Trans Neural Syst Rehabil Eng. 2019;27(2):256–64.
Wang T, Bao Y, Hao H, Zhang X, Li S, **e Q, Lan N, Niu CM. Customization of synergy-based FES for post-stroke rehabilitation of upper-limb motor functions. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:3541–4.
Maguire CC, Sieben JM, De Bie RA. Movement goals encoded within the cortex and muscle synergies to reduce redundancy pre and post-stroke. The relevance for gait rehabilitation and the prescription of walking-aids. A literature review and scholarly discussion. Physiother Theor Pr. 2019;35(1):1–14.
Israely S, Leisman G, Carmeli E. Neuromuscular synergies in motor control in normal and poststroke individuals. Rev Neuroscience. 2018;29(6):593–612.
Ferrante S, Chia Bejarano N, Ambrosini E, Nardone A, Turcato AM, Monticone M, Ferrigno G, Pedrocchi A. A personalized multi-channel FES controller based on muscle synergies to support gait rehabilitation after stroke. Front Neurosci. 2016;10:425.
Ting LH, Chiel HJ, Trumbower RD, Allen JL, Mckay JL, Hackney ME, Kesar TM. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron. 2015;86(1):38–54.
Niu CM, Chou CH, Bao Y, Wang T, Gu L, Zhang X, Cui L, Xuan Z, Zhuang C, Li S, et al. A pilot study of synergy-based FES for upper-extremity poststroke rehabilitation. Neurosci Lett. 2022;780: 136621.
Zhuang C, Marquez JC, Qu HE, He X, Lan N. A neuromuscular electrical stimulation strategy based on muscle synergy for stroke rehabilitation. I Ieee Embs C Neur E. 2015:816–19.
Ranzani R, Lambercy O, Metzger JC, Califfi A, Regazzi S, Dinacci D, Petrillo C, Rossi P, Conti FM, Gassert R. Neurocognitive robot-assisted rehabilitation of hand function: a randomized control trial on motor recovery in subacute stroke. J Neuroeng Rehabil. 2020;17(1):115.
Lin Y, Li QY, Qu Q, Ding L, Chen Z, Huang F, Hu S, Deng W, Guo F, Wang C, et al. Comparative effectiveness of robot-assisted training versus enhanced upper extremity therapy on upper and lower extremity for stroke survivors: a multicentre randomized controlled trial. J Rehabil Med. 2022;54:jrm00314.
Perini G, Bertoni R, Thorsen R, Carpinella I, Lencioni T, Ferrarin M, Jonsdottir J. Sequentially applied myoelectrically controlled FES in a task-oriented approach and robotic therapy for the recovery of upper limb in post-stroke patients: A randomized controlled pilot study. Technol Health Care. 2021;29(3):419–29.
Zhang XH, Gu T, Liu XW, Han P, Lv HL, Wang YL, **ao P. The effect of transcranial direct current stimulation and functional electrical stimulation on the lower limb function of stroke patients. Front Neurosci. 2021;15: 685931.
Fruhauf AMA, Politti F, Dal Corso S, Costa GC, Teodosio ADC, Silva SM, Correa JCF, Correa FI. Immediate effect of transcranial direct current stimulation combined with functional electrical stimulation on activity of the tibialis anterior muscle and balance of individuals with hemiparesis stemming from a stroke. J Phys Ther Sci. 2017;29(12):2138–46.
Yang S, Chen J, Guo Y, Teng Y, Liu T, Ying R, He Z, Wu J, Yu SG, Zeng F. Comparison of Taiji and aerobic exercise for functional constipation: study protocol for a randomised controlled neuroimaging trial. BMJ Open. 2019;9(8): e031089.
Takebayashi T, Takahashi K, Amano S, Gosho M, Sakai M, Hashimoto K, Hachisuka K, Uchiyama Y, Domen K. Robot-assisted training as self-training for upper-limb hemiplegia in chronic stroke: a randomized controlled trial. Stroke. 2022;53(7):2182–91.
Khaw J, Subramaniam P, Abd Aziz NA, Ali Raymond A, Wan Zaidi WA, Ghazali SE. Current Update on the Clinical Utility of MMSE and MoCA for Stroke Patients in Asia: A Systematic Review. Int J Environ Res Public Health. 2021;18(17):8962.
Berger VW, Bour LJ, Carter K, Chipman JJ, Everett CC, Heussen N, Hewitt C, Hilgers RD, Luo YA, Renteria J, et al. A roadmap to using randomization in clinical trials. BMC Med Res Methodol. 2021;21(1):168.
Minelli C, Bazan R, Pedatella MTA, Neves LO, Cacho RO, Magalhaes S, Luvizutto GJ, Moro CHC, Lange MC, Modolo GP, et al. Brazilian academy of neurology practice guidelines for stroke rehabilitation: part I. Arq Neuropsiquiatr. 2022;80(6):634–52.
Minelli C, Luvizutto GJ, Cacho RO, Neves LO, Magalhaes S, Pedatella MTA, Mendonca LIZ, Ortiz KZ, Lange MC, Ribeiro PW, et al. Brazilian practice guidelines for stroke rehabilitation: Part II. Arq Neuropsiquiatr. 2022;80(7):741–58.
Kapadia N, Moineau B, Popovic MR. Functional electrical stimulation therapy for retraining reaching and gras** after spinal cord injury and stroke. Front Neurosci. 2020;14:718.
Trigili E, Grazi L, Crea S, Accogli A, Carpaneto J, Micera S, Vitiello N, Panarese A. Detection of movement onset using EMG signals for upper-limb exoskeletons in reaching tasks. J Neuroeng Rehabil. 2019;16(1):45.
Shourijeh MS, Flaxman TE, Benoit DL. An approach for improving repeatability and reliability of non-negative matrix factorization for muscle synergy analysis. J Electromyogr Kinesiol. 2016;26:36–43.
Hirai T, Jiang Y, Sugi M, Togo S, Yokoi H. Investigation of motor point shift and contraction force of triceps brachii for functional electrical stimulation. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:6330–3.
Khodamipour G, Khorashadizadeh S, Farshad M. Adaptive formation control of leader-follower mobile robots using reinforcement learning and the Fourier series expansion. ISA Trans. 2023;138:63–73.
Frykberg GE, Grip H, Alt Murphy M. How many trials are needed in kinematic analysis of reach-to-grasp?-A study of the drinking task in persons with stroke and non-disabled controls. J Neuroeng Rehabil. 2021;18(1):101.
Furmanek MP, Mangalam M, Yarossi M, Lockwood K, Tunik E. A kinematic and EMG dataset of online adjustment of reach-to-grasp movements to visual perturbations. Scientific data. 2022;9(1):23.
Chen P, Liu TW, Kwong PWH, Lai CKY, Chung RCK, Tsoh J, Ng SSM. Bilateral transcutaneous electrical nerve stimulation improves upper limb motor recovery in stroke: a randomized controlled trial. Stroke. 2022;53(4):1134–40.
Hsieh YW, Wu CY, Lin KC, Chang YF, Chen CL, Liu JS. Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke. 2009;40(4):1386–91.
Kwakkel G, Van Wegen E, Burridge JH, Winstein CJ, van Dokkum L, Alt Murphy M, Levin MF, Krakauer JW. Standardized measurement of quality of upper limb movement after stroke: Consensus-based core recommendations from the Second Stroke Recovery and Rehabilitation Roundtable. Int J Stroke. 2019;14(8):783–91.
Alt Murphy M, Willen C, Sunnerhagen KS. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil Neural Repair. 2011;25(1):71–80.
Kwakkel G, van Wegen EEH, Burridge JH, Winstein CJ, van Dokkum LEH, Alt Murphy M, Levin MF, Krakauer JW, group A. Standardized measurement of quality of upper limb movement after stroke: consensus-based core recommendations from the second stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. 2019;33(11):951–8.
Alt Murphy M, Murphy S, Persson HC, Bergström UB, Sunnerhagen KS. Kinematic analysis using 3D motion capture of drinking task in people with and without upper-extremity impairments. J Vis Exp. 2018;133:57228.
Qiu Q, Fluet GG, Patel J, Iyer S, Karunakaran K, Kaplan E, Tunik E, Nolan KJ, Merians AS, Yarossi M, et al. Evaluation of changes in kinematic measures of three dimensional reach to grasp movements in the early subacute period of recovery from stroke. Annu Int Conf IEEE Eng Med Biol Soc. 2022;2022:5107–10.
Wagner JM, Rhodes JA, Patten C. Reproducibility and minimal detectable change of three-dimensional kinematic analysis of reaching tasks in people with hemiparesis after stroke. Phys Ther. 2008;88(5):652–63.
Hordacre B, Goldsworthy MR, Welsby E, Graetz L, Ballinger S, Hillier S. Resting state functional connectivity is associated with motor pathway integrity and upper-limb behavior in chronic stroke. Neurorehabil Neural Repair. 2020;34(6):547–57.
Acknowledgements
Not applicable.
Funding
This study was supported by grants through the Excellent Young Medical Talents Training Program of the Health Commission of Pudong New District (PWRq2020-13) and the Youth Research Project of the Shanghai Municipal Health Commission (20214Y0285).
Author information
Authors and Affiliations
Contributions
HX Z, RS Y, ZZ Z, MH L, LHZ S, KL L, and J H contributed to the study design and protocol. HX Z and J H were involved in drafting the manuscript or revising it for important intellectual comment. ZZ Z, HX Z and MH L contributed to the design of the synergy-based FES device. All authors reviewed and approved the final manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics and dissemination Ethics approval(2023-7th-HIRB-034) was obtained from the Ethics Committee of the Research Ethics Committee of the Seventh People's Hospital of Shanghai University of Traditional Chinese Medicine. All participants will sign the informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents*.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Hx., Hu, J., Yun, Rs. et al. Synergy-based functional electrical stimulation and robotic-assisted for retraining reach-to-grasp in stroke: a study protocol for a randomized controlled trial. BMC Neurol 23, 324 (2023). https://doi.org/10.1186/s12883-023-03369-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03369-2