Abstract
Background
Late-stage Parkinson’s disease (PD) often presents with neuropsychiatric symptoms such as dementia, psychosis, excessive daytime sleepiness, apathy, depression, and anxiety. However, neuropsychiatric symptoms are the cardinal features of Creutzfeldt–Jakob disease (CJD), raising the possibility that CJD may be an overlooked condition when it accompanies late-stage PD.
Case presentation
We describe a female autopsy case of PD with a typical clinical course of 17 years, in which CJD overlapped with PD during the final year of the patient’s life. The patient died aged 85 years. Neuropathological features included widespread Lewy body-related α-synucleinopathy predominantly in the brainstem and limbic system, as well as the typical pathology of methionine/methionine type 1 CJD in the brain.
Conclusions
Our case demonstrates the clinicopathological co-occurrence of PD and CJD in a sporadic patient. The possibility of mixed pathology, including prion pathology, should be taken into account when neuropsychiatric symptoms are noted during the disease course of PD.
Similar content being viewed by others
Background
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease, affecting more than 2% of people aged 65 and over [1]. The clinical features of PD consist of motor dysfunction, including bradykinesia, resting tremor, and rigidity, as well as non-motor symptoms, such as sleep disorders, mood disorders, sensory symptoms, olfactory dysfunction, dysautonomia, cognitive impairment, and dementia. The pathological hallmarks of PD are a marked loss of dopaminergic neurons in the substantia nigra pars compacta, which causes dopamine deficiency in the striatum, and the presence of intracytoplasmic eosinophilic inclusions known as Lewy bodies (LBs), with α-synuclein-immunoreactive neuronal pathology in the remaining neurons.
Creutzfeldt–Jakob disease (CJD) is a fatal neurodegenerative condition that is clinically characterized by rapidly progressive dementia, myoclonus, and ataxia. In terms of its pathology, the brain takes on a spongy appearance with abnormal prion protein (PrP) deposition. CJD occurs across all human populations, with an incidence of about 1.5 cases per million individuals per year [2]. CJD principally occurs in the age range of 50–80 years, although it is likely that the disease is clinically overlooked in the population older than 80 years [2].
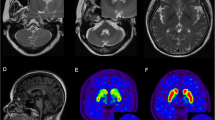
PD and CJD are clinically and neuropathologically distinct. Survival after a diagnosis of CJD is typically less than 1 year [2, 3], whereas that for PD may be 10–20 years [4, 5]. Here, we report a Japanese autopsy case of PD with a clinical course of 17 years who later developed rapidly progressive CJD. Recent advances in immunohistochemical methods have revealed a high frequency of multiple pathologies in single cases [6, 8] score was 12/30 in May 2019. In June 2019, myoclonus in the face and extremities were noted in addition to the exacerbation of generalized rigidity and bradykinesia; this led to nasogastric tube feeding. She had severe dementia and her Hoehn and Yahr stage was V, with no response to levodopa at 900 mg/day for 3 weeks. After a few months of unfavorable clinical evolution, the patient developed akinetic mutism in August 2019. Deep tendon reflexes were normal and no Babinski sign was elicited. Magnetic resonance imaging of the brain using diffusion-weighted imaging (MR-DWI) revealed extensive cortical ribbon-like and basal ganglia hypersignals, which were strongly consistent with CJD (Fig. 1). An electroencephalogram (EEG) was abnormal and showed diffuse slow waves with periodic synchronous discharges (PSD). Cerebrospinal fluid (CSF) examination [9] revealed elevation of 14-3-3 protein (>500 μg/mL) and total tau (>2,200 pg/mL). Real-time quaking-induced conversion (RT-QuIC) for PrP assays were positive using CSF [10]. The patient died of pneumonia in April 2020. The autopsy was limited to the brain.
Neuropathological findings
An autopsy was performed 15 hours after death. The brain weighed 1,000 g before fixation. A detailed description of neuropathological examination methods is provided in Supplementary Information (Additional file 1). Macroscopic examination revealed severe diffuse atrophy of both the cerebrum and cerebellum (Fig. 2a, b). The hippocampus was relatively spared but mildly atrophic. The olfactory bulb showed thinning. Depigmentation was evident in the substantia nigra and locus ceruleus (Fig. 2c, d). Microscopic examination revealed widespread, severe neuronal loss and gliosis, with ballooned neurons in the cerebral cortex. Fine vacuole-type spongiform changes were detected in all layers of the cerebral cortex that were examined (Fig. 2e). The entorhinal and transentorhinal cortices showed severe spongiform changes and neuronal loss, whereas the hippocampus and subiculum showed minimal changes. The striatum and thalamus, particularly in the medial part, showed apparent spongiform changes. The globus pallidus and subthalamic nucleus were generally preserved. In the cerebellum, the molecular layer showed mild spongiform changes; neuronal loss was severe in the granule cell layer and mild to moderate in the Purkinje cell layer (Fig. 2f). In the brainstem, the locus ceruleus and substantia nigra showed severe neuronal loss and gliosis, while such changes were not apparent in the inferior olivary nucleus. Immunostaining for PrP revealed synaptic-type PrP deposition in all layers of the cerebral cortex (Fig. 2g) as well as in the striatum, thalamus, and cerebellar molecular and granular cell layers. The hippocampus, subiculum, globus pallidus, dentate nucleus, substantia nigra, and inferior olivary nucleus also showed very mild to mild synaptic-type PrP deposition. No plaque-type PrP deposition was observed in the regions mentioned above. PrP deposition was not observed in the cerebral or cerebellar white matter.
Histopathology. Diffuse marked cerebral atrophy (a, b) and depigmentation of the substantia nigra (c) and locus ceruleus (d) are shown. Post-mortem tissue staining of the frontal cortex (e, g), cerebellar cortex (f), Edinger–Westphal nucleus (h), substantia nigra (i), and cornu ammonis 2 (j) is also shown. (e, f, h) Hematoxylin and eosin staining; (g) prion immunostaining; (i, j) phosphorylated α-synuclein immunostaining. Scale bars: a–d, 1 cm; e–g, j, 50 μm; h, i, 20 μm)
LB-related α-synucleinopathy (i.e., LBs, Lewy neurites, and neuronal intracytoplasmic inclusions that are immunoreactive for phosphorylated α-synuclein) was also distributed throughout the brainstem, including in the dorsal motor nucleus of the vagus nerve, locus ceruleus, substantia nigra, and Edinger–Westphal nucleus. It was also present in the nucleus basalis of Meynert and the limbic systems, including in the olfactory bulb, amygdala, hippocampus, and transentorhinal and cingulate cortices (Fig. 2h–j). Only a few phosphorylated α-synuclein-immunoreactive neuronal intracytoplasmic inclusions were observed in the neocortex.
Furthermore, scattered tufted astrocytes, globose-type neurofibrillary tangles, and coiled bodies that were immunoreactive for 4-repeat tau but not 3-repeat tau were observed in the inferior olivary nucleus, substantia nigra, red nucleus, superior colliculus, and periaqueductal gray matter of the midbrain, as well as in the subthalamic nucleus, putamen, globus pallidus, and precentral gyrus.
The level of Alzheimer’s disease-related neuropathological changes corresponded to “low” according to the National Institute on Aging-Alzheimer’s Association classification [11] (Thal phase for amyloid β plaques: 3 [12], Consortium to Establish a Registry for Alzheimer's Disease score: B [13], Braak NFT stage: II [14]). Very few phosphorylated TAR DNA-binding protein 43 (TDP-43)-immunoreactive neurites were observed in the uncus of the anterior hippocampus. No argyrophilic grains were identified.
PrP gene polymorphism and western blot studies
Gene analysis was done using genomic DNA extracted from the autopsied brain. PCR direct sequencing for the PRNP gene that encodes PrP revealed no mutations. The reference sequence used for the PRNP was NM_000311.5. Polymorphic codons showed methionine homozygosity at codon 129 and glutamate homozygosity at codon 219. Immunoblot analysis using anti-PrP antibody (3F4, Signet, Dedham, MA, USA) on a homogenized brain sample disclosed a type 1 pattern (Fig. 3; Supplementary Information for the original, unprocessed full-length version in Additional file 2).
Discussion and conclusions
In the literature, the clinical and pathological features of six CJD patients with LBs have been described previously (Table 1) [15,16,17,18,19,20]. Although the clinical sequence of illness is variable in CJD, a change in mental status usually occurs before motor symptoms become apparent. Only one case has previously been reported in which PD preceded CJD [15]; the initial clinical diagnoses of the other five cases were Alzheimer’s disease [16], mood disorder [17], CJD [18], mild cognitive impairment [19], and visual hallucination/delusion [20]. Because the interval between PD onset and CJD onset in the previously reported case was 2.5 years, the present case is—to the best of our knowledge—the first report of a case of PD with a typical long clinical course who later developed neuropathologically confirmed CJD. Given that neuropsychiatric symptoms represent milestones of late stages of PD, usually occurring 10-15 years after onset of parkinsonism [21], the short interval of 2.5 years in the previously reported case would suggest a high possibility of coexisting neuropsychiatric disorders such as CJD. However, the long course of PD in our patient meant that the diagnosis of CJD was relatively difficult because her neuropsychiatric symptoms could also be regarded as non-motor symptoms of the later stages of PD [22]. Because changes in mental status are common to both of these conditions, identifying CJD in patients with late-stage PD can prove challenging. There have been no systematic attempts to estimate the incidence of their co-occurrence in single patients; however, assuming a prevalence rate of 130 per 100,000 for PD [1] and an incidence rate of 0.15 per 100,000 per year for CJD [2], their co-occurrence would be expected in two people per billion per year. Nevertheless, only two cases (including our patient; Table 1) have been reported to have developed CJD after an established clinical diagnosis of PD, suggesting that a number of such cases may simply go unreported or be overlooked. In contrast to the time interval between onset of neuropsychiatric symptoms and death in PD (3-5 years [5]), the interval is so short in CJD (< 1 year [2, 3]) that usually neither CJD patients nor their families have time to reflect, arrange affairs, or fully anticipate the disease progress and mortal outcome. Therefore, arriving at the correct diagnosis as early as possible would be helpful to patients and families. We hope that the present case report will encourage physicians to add CJD to their list of differential diagnoses for neuropsychiatric symptoms during the disease course of PD, particularly in its later stages. It is noteworthy that in a recent post-mortem series of sixteen cases with dementia with Lewy bodies (DLB) clinically suspected of CJD, the presence of MR-DWI hyperintensities and/or PSD on an EEG was more likely to distinguish CJD from DLB compared to that of clinical signs such as myoclonus or ataxia [23]. In this context, MR-DWI and EEG would be tests of choice for PD patients presenting with neuropsychiatric symptoms as well as myoclonus or ataxia. In fact, ataxia, one of the characteristic features of CJD [2], was clinically undetectable in our patient due to the severe dementia and akinesia in spite of the presence of pathologically extensive cerebellar lesions, while testing MR-DWI and EEG lead to the clinical diagnosis of CJD.
Despite the usual limitations of cross-sectional assessments of end-stage neuropathology, we also found focal scattered tufted astrocytes, globose-type neurofibrillary tangles, and coiled bodies that were immunoreactive for 4-repeat tau in our patient; this corresponds to the early stages of progressive supranuclear palsy [24]. Notably, the coexistence of the deposition of proteins such as amyloid-β, tau, and/or TDP-43 in brains with CJD with LBs has been reported in five out of the seven published autopsy cases (including our patient; Table 1), thus emphasizing the importance of mixed pathologies in clinico-neuropathological studies of CJD as well as in older individuals. Indeed, it has been noted that the comorbidity of neurodegenerative diseases occurs more frequently than would be expected from the epidemiological data for each disease [25,26,27]. Molecular cross-talk among misfolded proteins through cross-seeding might explain the frequent finding of mixed pathologies [28]. Although a causative link between PD and CJD remains poorly understood, the possibility of cross-seeding cannot be discounted, warranting biochemical diagnoses such as quantification and RT-QuIC for α-synuclein and PrP [28]. In contrast, double immunohistochemistry for phosphorylated α-synuclein and PrP exhibited no explicit colocalization in the substantia nigra in our case (data not shown). Moreover, PrP was stained as the synaptic pattern in the brain regions examined as described above, whereas phosphorylated α-synuclein in intracellular structures including the cytoplasm and neurites. Elucidating whether the coexistence of proteinopathies in single patients is coincidental or not could aid to understand the etiology of the comorbidity of neurodegenerative diseases.
In conclusion, the present study demonstrated the clinicopathological co-occurrence of PD and CJD in a sporadic patient. The possibility of mixed pathology should be taken into account when neuropsychiatric symptoms develop, even in late-stage PD.
Availability of data and materials
The data that support the findings presented in this study are available from the corresponding author upon reasonable request.
Abbreviations
- CJD:
-
Creutzfeldt–Jakob disease
- LB:
-
Lewy body
- PD:
-
Parkinson’s disease
- PrP:
-
prion protein
- TDP-43:
-
TAR DNA-binding protein 43
References
Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–90. https://doi.org/10.1002/mds.25945.
LS. H. Prion diseases. In: Lewis ED MS, Rowland LP, eds, editor. Merritt’s Neurology, thirteenth ed. Pennsylvania: Lippincott Williams & Wilkins; 2015. p. 584–92.
Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46(2):224–33. https://doi.org/10.1002/1531-8249(199908)46:2<224::AID-ANA12>3.0.CO;2-W.
Nakashima K, Maeda M, Tabata M, Adachi Y, Kusumi M, Ohshiro H. Prognosis of Parkinson's disease in Japan. Tottori University Parkinson's Disease Epidemiology (TUPDE) Study Group. Eur Neurol. 1997;38(Suppl 2):60–3. https://doi.org/10.1159/000113485.
Kempster PA, O'Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain. 2010;133(Pt 6):1755–62. https://doi.org/10.1093/brain/awq059.
Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171–86. https://doi.org/10.1007/s00401-017-1717-7.
Robinson JL, Lee EB, **e SX, Rennert L, Suh E, Bredenberg C, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181–93. https://doi.org/10.1093/brain/awy146.
Hasegawa K. An investigation of dementia rating scale for the elderly. Seishinigaku. 1974;16:965–9.
Satoh K, Shirabe S, Eguchi H, Tsu**o A, Eguchi K, Satoh A, et al. 14-3-3 protein, total tau and phosphorylated tau in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease and neurodegenerative disease in Japan. Cell Mol Neurobiol. 2006;26(1):45–52. https://doi.org/10.1007/s10571-006-9370-z.
Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 2011;17(2):175–8. https://doi.org/10.1038/nm.2294.
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. https://doi.org/10.1007/s00401-011-0910-3.
Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–800. https://doi.org/10.1212/wnl.58.12.1791.
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479-86. https://doi.org/10.1212/WNL.41.4.479.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. https://doi.org/10.1007/BF00308809.
Ezrin-Waters C, Resch L, Lang AE. Coexistence of idiopathic Parkinson's disease and Creutzfeldt-Jakob disease. Can J Neurol Sci. 1985;12(3):272–3. https://doi.org/10.1017/s0317167100047156.
Iida T, Doh-ura K, Kawashima T, Abe H, Iwaki T. An atypical case of sporadic Creutzfeldt-Jakob disease with Parkinson's disease. Neuropathology. 2001;21(4):294–7. https://doi.org/10.1046/j.1440-1789.2001.00407.x.
Vital A, Canron MH, Gil R, Hauw JJ, Vital C. A sporadic case of Creutzfeldt-Jakob disease with beta-amyloid deposits and alpha-synuclein inclusions. Neuropathology. 2007;27(3):273–7. https://doi.org/10.1111/j.1440-1789.2007.00755.x.
Haraguchi T, Terada S, Ishizu H, Sakai K, Tanabe Y, Nagai T, et al. Coexistence of Creutzfeldt-Jakob disease, Lewy body disease, and Alzheimer's disease pathology: an autopsy case showing typical clinical features of Creutzfeldt-Jakob disease. Neuropathology. 2009;29(4):454–9. https://doi.org/10.1111/j.1440-1789.2008.00964.x.
Fernandez-Vega I, Ruiz-Ojeda J, Juste RA, Geijo M, Zarranz JJ, Sanchez Menoyo JL, et al. Coexistence of mixed phenotype Creutzfeldt-Jakob disease, Lewy body disease and argyrophilic grain disease plus histological features of possible Alzheimer's disease: a multi-protein disorder in an autopsy case. Neuropathology. 2015;35(1):56–63. https://doi.org/10.1111/neup.12150.
Vita MG, Tiple D, Bizzarro A, Ladogana A, Colaizzo E, Capellari S, et al. Patient with rapidly evolving neurological disease with neuropathological lesions of Creutzfeldt-Jakob disease, Lewy body dementia, chronic subcortical vascular encephalopathy and meningothelial meningioma. Neuropathology. 2017;37(2):110–5. https://doi.org/10.1111/neup.12343.
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–44. https://doi.org/10.1002/mds.21956.
Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol. 2012;8(8):435–42. https://doi.org/10.1038/nrneurol.2012.126.
Geut H, Vergouw LJM, Galis Y, Ingrassia A, de Jong FJ, Quadri M, et al. Neuropathological and genetic characteristics of a post-mortem series of cases with dementia with Lewy bodies clinically suspected of Creutzfeldt-Jakob's disease. Parkinsonism Relat Disord. 2019;63:162–8. https://doi.org/10.1016/j.parkreldis.2019.02.011.
Nogami A, Yamazaki M, Saito Y, Hatsuta H, Sakiyama Y, Takao M, et al. Early Stage of Progressive Supranuclear Palsy: A Neuropathological Study of 324 Consecutive Autopsy Cases. J Nippon Med Sch. 2015;82(6):266–73. https://doi.org/10.1272/jnms.82.266.
Forrest SL, Kim JH, De Sousa C, Cheong R, Crockford DR, Sheedy D, et al. Coexisting Lewy body disease and clinical parkinsonism in amyotrophic lateral sclerosis. Eur J Neurol. 2021;28(7):2192–9. https://doi.org/10.1111/ene.14849.
Araki K, Sumikura H, Matsudaira T, Sugiura A, Takao M, Murayama S, et al. Progressive supranuclear palsy and Parkinson's disease overlap: A clinicopathological case report. Neuropathology. 2016;36(2):187–91. https://doi.org/10.1111/neup.12259.
Fujita K, Matsubara T, Miyamoto R, Sumikura H, Takeuchi T, Maruyama Saladini K, et al. Co-morbidity of progressive supranuclear palsy and amyotrophic lateral sclerosis: a clinical-pathological case report. BMC Neurol. 2019;19(1):168. https://doi.org/10.1186/s12883-019-1402-7.
Soto C, Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci. 2018;21(10):1332–40. https://doi.org/10.1038/s41593-018-0235-9.
Acknowledgments
The authors would like to thank the patient and her daughters for their generosity and goodwill, Dr. Iván Fernández-Vega for providing clinical data on their study, and Mr. Yutaka Koga, Ms. Mieko Harada, Ms. Kyoko Okamoto, Ms. Nobuko Naoi, and Ms. Sachiko Imai for providing technical assistance.
Funding
This work was supported by Grants-in Aid from the Research Committee of CNS Degenerative Diseases, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health, Labour and Welfare Sciences Research Grants, the Ministry of Health, Labour and Welfare, Japan (YS), AMED Grant Number JP18dm0107103 (YS), and MEXT/JSPS KAKENHI Grant Number JP 16H06277 (YS). The funding body did not play any role in the design of the study; the collection, analysis, or interpretation of data; or the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
SK analyzed and interpreted the data and drafted the manuscript. TM, AT, and YS analyzed and interpreted the data and revised the manuscript. TT and RS revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to publication
Written consent was obtained from the patient’s family for publication of this case report and any accompanying data.
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of Eisei Hospital under approval number 2021-005. The patient’s family provided written informed consent prior to investigation.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Procedures for neuropathological examination.
Additional file 2.
Original, unprocessed version of immunoblot for PrP.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kubo, Si., Matsubara, T., Taguchi, T. et al. Parkinson’s disease with a typical clinical course of 17 years overlapped by Creutzfeldt–Jakob disease: an autopsy case report. BMC Neurol 21, 480 (2021). https://doi.org/10.1186/s12883-021-02504-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-021-02504-1