Abstract
Background
Malnutrition is common in critically ill patients, but nutrition status in critically ill patients with acute kidney injury (AKI) has been poorly studied. Our study aimed to investigate the relationship between malnutrition risk and the occurrence and prognosis of AKI in elderly patients in the intensive care unit (ICU).
Methods
Data were extracted from the Bei**g Acute Kidney Injury Trial (BAKIT). A total of 1873 elderly patients were included and compared according to the clinical characteristics of AKI and non-AKI groups, and those of survivors and non-survivors of AKI in this study. Receiver operating characteristic (ROC) curves were used to analyse the predictive value of the modified Nutrition Risk in Critically Ill (mNUTRIC) score for the occurrence and 28-day prognosis of AKI. Multivariate Cox regression analysis was used to evaluate the effect of the mNUTRIC score on the 28-day mortality in AKI patients.
Results
Compared with the non-AKI group, AKI patients had higher mNUTRIC scores, and non-survivors had higher mNUTRIC scores than survivors in AKI population. Moreover, multivariate Cox regression showed that 28-day mortality in AKI patients increased by 9.8% (95% CI, 1.018-1.184) for every point increase in the mNUTRIC score, and the mNUTRIC score had good predictive ability for the occurrence of AKI and 28-day mortality in AKI patients. The mortality of AKI patients with mNUTRIC > 4 was significantly increased.
Conclusions
The elderly patients are at high risk of malnutrition, which affects the occurrence and prognosis of AKI. Adequate attention should be given to the nutritional status of elderly patients.
Trial registration
This study was registered at www.chictr.org.cn (registration number Chi CTR-ONC-11001875) on 14 December 2011.
Similar content being viewed by others
Background
Malnutrition is highly prevalent in the elderly population with acute kidney injury (AKI) [1, 2], which increases nosocomial mortality [3, 4]. Moreover, patients with malnutrition are proven to have an increased risk of AKI [5]. Nutritional status assessment is critical to identify elderly patients who may easily to suffer from AKI and are at risk of mortality [6]. Traditional nutritional screening tools, including weight loss, food intake reduction and laboratory values, are not reliable in AKI patients who cannot provide these details and may have water electrolyte disorders [7]. In addition, elderly patients with AKI in the intensive care unit (ICU) often suffer from volume resuscitation, resulting in rapid weight gain and even tissue edema. Body mass index, skin fold thickness and other data cannot accurately reflect the nutritional status of AKI patients.
The Nutrition Risk in Critically Ill (NUTRIC) score was proposed by Canadian scholar Heyland in 2011 [8], which is a nutritional assessment tool specifically designed for critically ill patients. The NUTRIC score includes 6 items: age, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, number of comorbidities, length of hospital stay before admission to the ICU and interleukin-6 (IL-6) level. Each item is scored between minimum 0 and maximum 3 points according to its importance. When IL-6 cannot be obtained routinely, the modified NUTRIC (mNUTRIC) score is also acceptable [9]. Nutritional risk is low when the mNUTRIC score is 0 ~ 4, and high when the mNUTRIC score is 5 ~ 9 [8, 9]. The mNUTRIC score is related to adverse clinical outcomes (death and long duration of mechanical ventilation) [9,10,11,12].
Previous studies using albumin [13], prealbumin [14], body mass index (BMI) [15], Controlling Nutritional Status score (CONUT) [2], Nutritional Risk Screening 2002 (NRS-2002) [5, 6] and other indicators have found a higher mortality in AKI patients with malnutrition risk, but there is no report on the relationship between mNUTRIC score and AKI in the elderly population. The purpose of this study was to investigate the effect of the mNUTRIC score on the development and prognosis of AKI in the elderly ICU population.
Materials and methods
Study population
This study was a secondary analysis of the Bei**g Acute Kidney Injury Trial (BAKIT) [16], which is a prospective, multicenter study that investigated the epidemiology of AKI in critically ill patients admitted to 30 ICUs at 28 tertiary hospitals in Bei**g, China, from March 1 to August 31, 2012 (for a complete list of these hospitals and the personnel responsible for data collection, please refer to Additional file). Patients over 18 years old were enrolled consecutively, and only first-time ICU admissions were considered in this study. Patients with end-stage chronic kidney disease, renal replacement therapy (RRT) before admission to the ICU, renal transplantation in the previous 3 months, hospitalization less than 24 hours or incomplete clinical data were excluded. According to the World Health Organization standard, the elderly is defined as older than 60 years old [17].
Data collection
Age, sex, BMI, admission date, admission diagnosis, comorbidities, organ failure, nephrotoxic drugs, baseline creatinine, APACHE II, SOFA, and the Simplified Acute Physiology Score II (SAPS II) score were recorded. Daily vital signs, laboratory data, urine output, use of vasoactive drugs, diuretics, and sepsis were continuously recorded for 10 days or until the patient was discharged from the ICU. The occurrence of AKI, length of mechanical ventilation (MV), RRT data and ICU length of stay (LOS) were also reported. The primary outcome was 28-day mortality.
We calculated the mNUTRIC score within the first day of ICU admission. Parameters for calculating the mNUTRIC score can be found in another article [8].
Definition of AKI
AKI was defined and classified according to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [18]. The calculation of baseline creatinine can be found in our previous paper [16].
Nutritional support
Nutritional support methods were based on the guidelines for enteral and parenteral nutrition issued by the Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) [19]. See our published article for details [12].
Statistical analysis
SPSS software (IBM Corp., Statistics for Windows, version 22.0, Armonk, NY, USA) was used for data analysis, A two-sided P values < 0.05 was considered statistically significant. After normality testing, continuous variables were expressed as mean and standard deviation (SD) or medians (M) and quartiles (Q1, Q3), and compared using the Student’s t test or Mann-Whitney U test. Categorical variables were expressed as percentages, and the chi-squared test was used for comparison.
The hazards model (Cox) was used to analyse the risk factors for 28-day mortality in elderly patients with AKI. Since age, SOFA and APACHE II scores were included in the mNUTRIC score, collinearity analysis was required. Due to the collinearity of mNUTRIC with age or APACHE II, variables considered in the multivariate analysis included BMI, SAPS II, SOFA, mNUTRIC, sepsis, RRT, and AKI grades.
The discriminatory ability of the mNUTRIC score for AKI occurrence and prognosis was evaluated by receiver operating characteristic (ROC) curve analysis, and the areas under the curve (AUCs) were calculated. Youden index was used to establish the optimal cut-off value, and sensitivity, specificity, positive predictive value and negative predictive value were also reported. The Hosmer-Lemeshow goodness-of-fit test was used to test the calibration of the scoring system.
Kaplan-Meier survival curves were used to compare the cumulative survival rates among the four groups: low nutritional risk plus non-AKI vs. low nutritional risk plus AKI vs. high nutritional risk plus non-AKI vs. high nutritional risk plus AKI.
Results
Study population
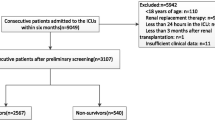
During the study period, 9049 patients were admitted consecutively, excluding unqualified cases, a total of 3107 patients were enrolled in the BAKIT study, and we previously published detail data on this population [16]. Of these patients, 1234 patients were excluded because they were younger than 60 years, leaving a final sample of 1873 patients for this study. A total of 1021 patients developed AKI, and 694 patients survived more than 28 days (Fig. 1). Among elderly patients with AKI, 333 were septic AKI, 331 were postoperative AKI, 85 were AKI due to hypovolemia, and 79 were AKI caused by drugs (including contrast agents). In the elderly population, 39.4% (738/1873) of patients had a higher nutritional risk according to the mNUTRIC score.
Baseline characteristics of the entire cohort and stratified data according to AKI occurrence are summarized in Table 1. The median age was 74 (Q1, Q3: 66, 81) years, and 60.4% were men. The all-cause 28-day mortality rate was 21.1% (396/1873), and the median ICU LOS was 5 (Q1, Q3: 2, 10) days. Among the included patients, the median BMI was 24 (Q1, Q3: 21, 26) Kg/m2, the median mNUTRIC score was 4 (Q1, Q3: 3, 5), the median APACHE II score was 16 (Q1, Q3: 12, 21), the median SAPS II was 38 (Q1, Q3: 30, 49) and the median SOFA score was 6 (Q1, Q3: 3, 9). MV was performed in 1215 (64.9%) patients, 802 patients (42.8%) were treated with vasopressors, and 180 patients (9.6%) underwent RRT.
There were statistically significant differences in age, sepsis, mNUTRIC, APACHE II, SAPS II, SOFA, admission category, ICU LOS and 28-day mortality between AKI and non-AKI patients.
Comparison of characteristics between survival and non-survival patients with AKI
Compared with the survivors, non-survivors were older, had lower BMI, higher nutritional risk, worse critical illness score, more use of mechanical ventilation and RRT, higher level of organ support, more prone to sepsis, and worse AKI grade. See Table 2. Among elderly patients with AKI, 54.0% (551/1021) were at higher nutritional risk according to the mNUTRIC score.
The predictive ability of mNUTRIC for the occurrence of AKI and poor outcomes
The ROC curve of the mNUTRIC score predicting AKI occurrence is shown in Fig. 2a. The cut-off value was > 4 with a sensitivity of 53.97% and specificity of 78.05%. The ROC curve of the mNUTRIC score for 28-day mortality in AKI patients is shown in Fig. 2b. The cut-off value was > 4 with a sensitivity of 76.45% and specificity of 56.63%. The Hosmer-Lemeshow test showed that the goodness of fit of the score was good, and the chi-square (P value) values were 7.753 (0.170) and 3.260 (0.660), respectively. Patients with high nutritional risk had a higher incidence of AKI and a poorer prognosis than those with low nutritional risk (Table 3).
Cox regression analyses of 28-day mortality in AKI patients
A Cox regression model was used to test the effect of the mNUTRIC score in predicting 28-day mortality in patients with AKI (Table 4). Because the mNUTRIC score is collinear with APACHE II or age, variables considered for Cox regression analysis included BMI, mNUTRIC score, SAPS II, SOFA, sepsis, RRT and AKI grade. Multivariable analysis showed that 28-day mortality increased by 9.8% (95% CI, 1.018-1.184) for every point increase in the mNUTRIC score. In addition to mNUTRIC, SAPS II, sepsis, and worse AKI grade were significantly associated with a higher risk of death in multivariable analysis.
Survival curve of 28-day mortality by the low or high of mNUTRIC scores in the AKI/non-AKI patients
The patients were divided into four groups according to the cut-off value of the mNUTRIC score and the presence or absence of AKI: low nutritional risk plus non-AKI vs. low nutritional risk plus AKI vs. high nutritional risk plus non-AKI vs. high nutritional risk plus AKI. Kaplan-Meier survival curves were used to compare the cumulative survival rates among the four groups (Fig. 3). The 28-day survival rate was significantly increased in patients with high nutritional risk and AKI. When patients had AKI and high nutritional risk, the 28-day mortality increased to 45.2% (Fig. 4).
Discussion
Due to the inflammatory response, surgery, trauma and other reasons, catabolism is significantly enhanced and anabolism is weakened in critically ill patients, resulting in increased nutritional risk. Critically ill patients experience the blow of the disease, and their immune function is suppressed. When combined with malnutrition, immune suppression is further aggravated, resulting in aggravated infection, delayed wound healing, acquired muscle weakness and difficulty in weaning, resulting in increased complications, including AKI and increased mortality. Malnutrition is common in the elderly population [20, 21], energy intake decreases as the body weakens with age, and age is an important factor for malnutrition. Some nutrition screening tools, such as NRS-2002 [22], Patient-Generated Subjective Global Assessment (PG-SGA) [23] and mNUTRIC score, all include age. Malnutrition is often found in patients with acute kidney injury (AKI) [3, 5, 24], and it is an independent risk factor for poor prognosis in critically ill patients [25, 26]. It affects the occurrence and development of AKI independently of non-nutritional factors, increases in-hospital mortality, prolongs hospitalization time and increases hospitalization expenses [5].
The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommended that all hospitalized patients with AKI should be screened for malnutrition [27]. However, due to the complex and multifactorial nature of malnutrition in patients with kidney diseases, the best tool to identify patients at high risk of malnutrition is still in dispute [1, 28]. The NUTRIC score was designed for the ICU population, and its performance for critically ill patients may be better than NRS-2002 [28, 29]. Our study used the mNUTRIC score as an assessment tool to evaluate the nutritional risk of elderly AKI patients and found that 551 (54.0%) of these patients had a higher nutritional risk in elderly AKI population. Our findings support the need to enhance the identification of malnutrition risk among elderly patients in the ICU. This may improve the risk stratification of patients and guide the prevention of AKI.
Our study found that older patients with higher nutritional risk were more likely to develop AKI than those with lower nutritional risk (74.7% vs. 41.4%) (Table 3). The predictive ability of the mNUTRIC score for the occurrence of AKI was good, but its sensitivity was low (Fig. 2). Similarly, another study showed that increased nutritional risk was independently associated with the presence of contrast-induced AKI (CI-AKI), and malnutrition assessment of elderly patients before diagnosis or coronary intervention may help clinicians identify patients with elevated risk for CI-AKI [1]. Wei et al. also found that moderate-severe malnutrition evaluated by the CONUT score is associated with a higher risk of contrast-associated AKI (CA-AKI) in elderly patients undergoing percutaneous coronary intervention (PCI) [2]. Recently, a retrospective propensity score matching study enrolled 46,549 inpatients and found that patients with NRS-2002 scores ≥3 had a higher incidence of AKI than normal nutritional patients, and the undernourished patients who developed AKI had a far worse prognosis than normal nutritional patients [5]. Early identification of patients with high nutritional risk and adequate nutritional support treatment to reduce the occurrence of AKI is very important to improve the prognosis of patients.
Malnutrition is common in critically ill patients and is closely related to the prognosis of AKI patients [13, 14]. However, the nutritional status of AKI patients is often ignored [21]. Accurately assessing the nutritional status of patients and providing nutritional support is still a challenging task in AKI treatment. Fiaccadori et al. conducted a study of 309 patients with AKI and found that 58% of patients had malnutrition, and severe malnutrition was associated with poor prognosis [3]. Another study also found that low calorie intake, high C-reactive protein level, edema and low nitrogen balance were significantly associated with the risk of death in AKI patients [4]. The risk of malnutrition assessed by the NRS-2002 helps to identify high-risk patients with AKI and mortality, and patients with acute coronary syndrome can benefit from further nutritional intervention and prevention of AKI [6]. A meta-analysis showed that protein-energy wasting (PEW) assessed using subjective global assessment (SGA) was associated with greater mortality risk (RR: 1.99, 95% CI: 1.36–2.91). Individual nutrition parameters, such as serum chemistry, body mass, muscle mass, and dietary intake, were not consistently associated with mortality in patients with AKI [30]. Our study showed that the mNUTRIC score was an effective tool to evaluate the prognosis of AKI patients. After adjusting for multiple risk factors, 28-day mortality in AKI patients increased by 9.8% (95% CI, 1.018-1.184) for every point increase in the mNUTRIC score.
Our study found that high nutritional risk patients assessed using the mNUTRIC score had a worse prognosis than low nutritional risk patients. When AKI was present, the mortality increased significantly (Fig. 3 and 4), which is consistent with other studies [5, 6, 31]. Under pathological conditions, the interaction between malnutrition and AKI is close and complex. For example, malnutrition may lead to AKI, which in turn is a harmful factor of malnutrition. Our research showed that high nutritional risk is closely related to AKI, and both contribute to the poor prognosis of patients. Li et al. [5] also found that there was a strong association between the NRS-2002 and AKI and that the risk of AKI changed in parallel with the NRS-2002 score. Both AKI and NRS-2002 scores ≥3 can worsen the prognosis.
Our study had several limitations: First, our investigation was limited to the risk factors available in the original database and did not record albumin, prealbumin, or total cholesterol, etc., although serum markers may not have good predictive ability [32]. Second, we did not differentiate the onset and duration of AKI, which may affect patient outcomes. Third, there was no dynamic nutritional assessment, which might have been more meaningful. Further studies are needed to determine the value of high nutritional risk in elderly patients with AKI.
Conclusions
High nutritional risk based on the mNUTRIC score is common in elderly patients with AKI and is a risk factor in poor prognoses. Early nutritional risk assessment and targeted treatment are recommended for critically ill patients, which may be of great significance in preventing the occurrence of AKI and improving the prognosis of patients.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to there was a confidentiality agreement signed with the partner hospitals, but available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- ICU:
-
Intensive care unit
- BAKIT:
-
The Bei**g Acute Kidney Injury Trial
- ROC:
-
Receiver operating characteristic curve
- mNUTRIC:
-
The modified Nutrition Risk in Critically Ill score
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- SAPS II:
-
Simplified Acute Physiology Score II
- SOFA:
-
Sequential Organ Failure Assessment
- BMI:
-
Body mass index
- CONUT:
-
Controlling Nutritional Status score
- RRT:
-
Renal replacement therapy
- MV:
-
Mechanical ventilation
- LOS:
-
Length of stay
- KDIGO:
-
Kidney Disease Improving Global Outcomes guidelines
- COPD:
-
Chronic obstructive pulmonary disease
- CI:
-
Confidence interval
- AUC:
-
The areas under the curve
- PG-SGA:
-
Patient-Generated Subjective Global Assessment
- ESPEN:
-
The European Society for Parenteral and Enteral Nutrition
- PEA:
-
Protein-energy wasting
References
Efe SC, Karagöz A, Doğan C, Bayram Z, Cakmak EO, Kalkan S, et al. Prognostic significance of malnutrition scores in elderly patients for the prediction of contrast-induced acute kidney injury. Int J Clin Pract. 2021;75(7):e14274.
Wei X, Chen H, You Z, Yang J, He H, He C, et al. Nutritional status and risk of contrast-associated acute kidney injury in elderly patients undergoing percutaneous coronary intervention. Clin Exp Nephrol. 2021;25(9):953–62.
Fiaccadori E, Lombardi M, Leonardi S, Rotelli CF, Tortorella G, Borghetti A. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol. 1999;10:581e93.
Berbel MN, de Goes CR, Balbi AL, Ponce D. Nutritional parameters are associated with mortality in acute kidney injury. Clinics (Sao Paulo). 2014;69(7):476e82.
Li C, Xu L, Guan C, Zhao L, Luo C, Zhou B, et al. Malnutrition screening and acute kidney injury in hospitalised patients: a retrospective study over a 5-year period from China. Br J Nutr. 2020;123:337e46.
Yu J, Li D, Jia Y, Li F, Jiang Y, Zhang Q, et al. Nutritional risk screening 2002 was associated with acute kidney injury and mortality in patients with acute coronary syndrome: insight from the REACP study. Nutr Metab Cardiovasc Dis. 2021;31(4):1121–8.
McCarthy MS, Phipps SC. Special nutrition challenges: current approach to acute kidney injury. Nutr Clin Pract. 2014;29(1):56–62.
Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268.
Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland D. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin Nutr. 2016;35(1):158–62.
Jeong DH, Hong SB, Lim CM, Koh Y, Seo J, Kim Y, et al. Comparison of accuracy of NUTRIC and modified NUTRIC scores in predicting 28-Day mortality in patients with Sepsis: a single center retrospective study. Nutrients. 2018;10(7).
Mukhopadhyay A, Henry J, Ong V, Leong CS, Teh AL, van Dam RM, et al. Association of modified NUTRIC score with 28-day mortality in critically ill patients. Clin Nutr. 2017;36(4):1143–8.
Wang N, Wang MP, Jiang L, Du B, Zhu B, ** XM. Association between the modified nutrition risk in critically ill (mNUTRIC) score and clinical outcomes in the intensive care unit: a secondary analysis of a large prospective observational study. BMC Anesthesiol. 2021;21(1):220.
Zorrilla-Vaca A, Mena GE, Ripolles-Melchor J, Lorente JV, Ramirez-Rodriguez JJM, Grant MC. Risk factors for acute kidney injury in an enhanced recovery pathway for colorectal surgery. Surg Today. 2021;51(4):537–44.
You ZB, Lin KY, Zheng WP, Lin CJ, Lin F, Guo TL, et al. Association of prealbumin levels with contrast-induced acute kidney injury in elderly patients with elective percutaneous coronary intervention. Clin Interv Aging. 2018;13:641–9.
Wang B, Li D, Gong Y, Ying B, Cheng B, Sun L. Body mass index is associated with the severity and all-cause mortality of acute kidney injury in critically ill patients: an analysis of a large critical care database. Biomed Res Int. 2021;2021:6616120.
Luo X, Jiang L, Du B, Wen Y, Wang MP, ** XM. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014;18:R144.
Amuthavalli Thiyagarajan J, Mikton C, Harwood RH, Gichu M, Gaigbe-Togbe V, Jhamba T, et al. The UN Decade of healthy ageing: strengthening measurement for monitoring health and wellbeing of older people. Age Ageing. 2022;51(7):afac147.
KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;17:1–138.
McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (a.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211.
Corish CA, Bardon LA. Malnutrition in older adults: screening and determinants. Proc Nutr Soc. 2019;78(3):372–9.
Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and Meta-analysis. Nutrients. 2017;9(8):829.
Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415–21.
Bauer J, Capra S, Ferguson M. Use of the scored patient-generated subjective global assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779–85.
Meyer D, Mohan A, Subev E, Sarav M, Sturgill D. Acute kidney injury incidence in hospitalized patients and implications for nutrition support. Nutr Clin Pract. 2020;35(6):987–1000.
Czapla M, Juárez-Vela R, Gea-Caballero V, Zieliński S, Zielińska M. The association between nutritional status and in-hospital mortality of COVID-19 in critically-ill patients in the ICU. Nutrients. 2021;13(10):3302.
Cattani A, Eckert IC, Brito JE, Tartari RF, Silva FM. Nutritional risk in critically ill patients: how it is assessed, its prevalence and prognostic value: a systematic review. Nutr Rev. 2020;78(12):1052–68.
Fiaccadori E, Sabatino A, Barazzoni R, Carrero JJ, Cupisti A, De Waele E, et al. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin Nutr. 2021;40(4):1644–68.
Majari K, Imani H, Hosseini S, Amirsavadkouhi A, Ardehali SH, Khalooeifard R. Comparison of modified NUTRIC, NRS-2002, and MUST scores in Iranian critically ill patients admitted to intensive care units: a prospective cohort study. JPEN J Parenter Enteral Nutr. 2021;45(7):1504–13.
Canales C, Elsayes A, Yeh DD, Belcher D, Nakayama A, McCarthy CM, et al. Nutrition risk in critically ill versus the nutritional risk screening 2002: are they comparable for assessing risk of malnutrition in critically ill patients? JPEN J Parenter Enteral Nutr. 2019;43(1):81–7.
Khor BH, Tiong HC, Tan SC, Abdul Rahman R, Abdul Gafor AH. Protein-energy wasting assessment and clinical outcomes in patients with acute kidney injury: a systematic review with Meta-analysis. Nutrients. 2020;12(9):2809.
Han M, Lee HW, Lee HC, Kim HJ, Seong EY, Song SH. Impact of nutritional index on contrast-associated acute kidney injury and mortality after percutaneous coronary intervention. Sci Rep. 2021;11(1):7123.
Gong Y, Ding F, Gu Y. Can serum nutritional related biomarkers predict mortality of critically ill older patients with acute kidney injury? Clin Interv Aging. 2019;14:1763–9.
Acknowledgments
The authors thank all members of the Bei**g Acute Kidney Injury Trial (BAKIT) work group (see Additional file) for participating in database management.
Funding
This study was supported by a grant from the Bei**g Municipal Science & Technology Commission, a government fund used to improve health-care quality (No. D101100050010058). It offered financial support for data collection.
Author information
Authors and Affiliations
Contributions
NW and PW conducted the primary data search, performed the statistical analysis, and drafted the manuscript. WL and LJ were responsible for the oversight of data management, manuscript reviewing and editing. MPW and BZ were involved in the data collection and the statistical analysis. XMX designed the study and helped to revise manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This project was approved by the institutional review board of the ethics committee of Fu **ng Hospital, Peking Union Medical College Hospital, Bei**g Tongren Hospital, Bei**g Tiantan Hospital, Bei**g Ditan Hospital, Bei**g Chaoyang Hospital, Peking University Third Hospital, Peking University First Hospital, Peking University People’s Hospital, China-Japan Friendship Hospital, The 309th Hospital of Chinese People’s Liberation Army, Bei**g Shijitan Hospital, Fuwai Hospital, Air Force General Hospital of Chinese People’s Liberation Army, First Affiliated Hospital of General Hospital of People’s Liberation Army, Navy General Hospital, Luhe Teaching Hospital, Bei**g Anzhen Hospital, Bei**g Hospital, General Hospital of Armed Police Forces, The General Hospital of People’s Liberation Army, Bei**g YouAn Hospital, Hua**n Hospital, Bei**g Shunyi Hospital, Bei**g Geriatric Hospital and Bei**g No.6 Hospital. We confirm that all methods were carried out in accordance with relevant guidelines and regulations. As an observational study, participants did not need written informed consent to participate. Therefore, we obtained the informed consent exemption of the above ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, N., Wang, P., Li, W. et al. Prognostic significance of malnutrition risk in elderly patients with acute kidney injury in the intensive care unit. BMC Nephrol 23, 335 (2022). https://doi.org/10.1186/s12882-022-02949-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02949-7