Abstract
Background
Pre-existing comorbidities have been linked to SARS-CoV-2 infection but evidence is sparse on the importance and pattern of multimorbidity (2 or more conditions) and severity of infection indicated by hospitalisation or mortality. We aimed to use a multimorbidity index developed specifically for COVID-19 to investigate the association between multimorbidity and risk of severe SARS-CoV-2 infection.
Methods
We used data from the UK Biobank linked to laboratory confirmed test results for SARS-CoV-2 infection and mortality data from Public Health England between March 16 and July 26, 2020. By reviewing the current literature on COVID-19 we derived a multimorbidity index including: (1) angina; (2) asthma; (3) atrial fibrillation; (4) cancer; (5) chronic kidney disease; (6) chronic obstructive pulmonary disease; (7) diabetes mellitus; (8) heart failure; (9) hypertension; (10) myocardial infarction; (11) peripheral vascular disease; (12) stroke. Adjusted logistic regression models were used to assess the association between multimorbidity and risk of severe SARS-CoV-2 infection (hospitalisation/death). Potential effect modifiers of the association were assessed: age, sex, ethnicity, deprivation, smoking status, body mass index, air pollution, 25‐hydroxyvitamin D, cardiorespiratory fitness, high sensitivity C-reactive protein.
Results
Among 360,283 participants, the median age was 68 [range 48–85] years, most were White (94.5%), and 1706 had severe SARS-CoV-2 infection. The prevalence of multimorbidity was more than double in those with severe SARS-CoV-2 infection (25%) compared to those without (11%), and clusters of several multimorbidities were more common in those with severe SARS-CoV-2 infection. The most common clusters with severe SARS-CoV-2 infection were stroke with hypertension (79% of those with stroke had hypertension); diabetes and hypertension (72%); and chronic kidney disease and hypertension (68%). Multimorbidity was independently associated with a greater risk of severe SARS-CoV-2 infection (adjusted odds ratio 1.91 [95% confidence interval 1.70, 2.15] compared to no multimorbidity). The risk remained consistent across potential effect modifiers, except for greater risk among older age. The highest risk of severe infection was strongly evidenced in those with CKD and diabetes (4.93 [95% CI 3.36, 7.22]).
Conclusion
The multimorbidity index may help identify individuals at higher risk for severe COVID-19 outcomes and provide guidance for tailoring effective treatment.
Similar content being viewed by others
Background
The novel coronavirus SARS-CoV-2 has led to a global pandemic of a complex, pneumonia-related illness (COVID-19). As of 29th September 2020, there have been 33,749,599 confirmed cases of COVID-19 and 1,009,829 deaths reported worldwide [1]. Many countries are still facing a devastating rise in the number of cases and deaths, whilst some are facing a second wave such as France, Spain, UK, with significant impacts on their healthcare systems. The risk of SARS-CoV-2 infection is based on the person-to-person transmission rate at which the virus is circulating in the community [2, 3]. Yet, while some individuals recover from the SARS-CoV-2 infection, others develop severe illness which may require intensive care in hospital or result in mortality.
Several potential factors have been postulated or associated with the increased risk of transmission and development of severe SARS-CoV-2 infection, such as age, sex, ethnicity, body mass index (BMI), smoking, deprivation, pre-existing comorbidities (particularly diabetes, hypertension and cardiovascular disease), cardiorespiratory fitness, 25‐hydroxyvitamin D level, air pollution and inflammation [4]. However, individuals with pre-existing health conditions have been found to have the highest risk of develo** severe SARS-CoV-2 infection, as shown in a number of studies from early research based in Wuhan [5] to recent studies across the globe [6,7,8]. Nevertheless, the majority of research to date has focused on pre-existing single chronic conditions [9], yet individuals often have multiple chronic conditions, known as multimorbidity. In view of the rapidly increasing prevalence of severe SARS-CoV-2 infection across the globe, clarifying the pattern and impact of multimorbidity (defined as 2 or more conditions) on the risk of severe SARS-CoV-2 infection has important clinical and public health implications which are not captured by a focus on single diseases [10].
There are a number of methods to define multimorbidity, which include both simple measures such as summing up the diseases and more complex measures using a weighted score such as the Charles Comorbidity Index. A previous study in UK Biobank examined the pattern of the most prevalent clusters of multimorbidity in those infected with SARS-CoV-2, using 36 different conditions to define multimorbidity [11], though this masked the most relevant conditions related to SARS-CoV-2 [12]. Moreover, three studies which assessed the risk of multimorbidity and COVID-19 had also used a broad range of conditions (ranging from 19 to 43 conditions) [13,14,15]. In contrast, the available evidence indicates that certain health conditions are associated with a much greater risk of severe SARS-CoV-2 infection linked with hospitalisation or mortality [5, 16].
To help clarify the evidence, in this study we aimed to firstly develop a multimorbidity index of the most commonly reported chronic conditions from the current literature for severe SARS-CoV-2 infection. The index was then used to investigate the patterns and association between multimorbidity and risk of severe SARS-CoV-2 infection and we further tested whether this association was modified by other potential risk factors.
Methods
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Additional File 1: Checklist S1) and following a pre-specified protocol (Application Number 36371) [17].
Study population
UK Biobank included half a million middle-aged adults recruited from 22 sites across England, Wales and Scotland with baseline measures collected between 2006 and 2010, from an in-person baseline interview at the UK Biobank centre (http://biobank.ctsu.ox.ac.uk/crystal/search.cgi). Written informed consent was obtained prior to data collection; UK Biobank was approved by the NHS National Research Ethics Service (16/NW/0274; ethics approval for UK Biobank studies) [18].
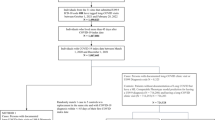
SARS-CoV-2 laboratory confirmed test results and death data for all-cause mortality from Public Health England were linked to the UK Biobank database [19]. Data were available between March 16 and July 26, 2020, restricted to those tested in a hospital (pillar 1), since this can be regarded as a proxy for hospitalisations for severe cases of the disease as suggested by the linkage methodology [19]. Our analytical sample included study participants from England as testing was available and linked, we therefore excluded study members from Scotland and Wales, participants who had an outpatient test positive for COVID-19 (pillar 2) as the outcome for SARS-CoV-2 infection was uncertain (i.e. recovered, hospitalised at a later stage, or died), those who died before March 16, 2020, and those with missing data (Additional file 1: Fig. S1).
Multimorbidity index
To use prior evidence on comorbidities that cluster together and that may act in combination to affect the risk of COVID-19, we carried out a literature search to identify the most common pre-existing comorbidities in patients with severe SARS-CoV-2 infection (Additional file 1: Table S1). The studies from the literature search were found to be very heterogeneous, therefore it was not possible to do a quantitative meta-analysis of pooled results [5,6,7,8, 20,21,22,23,24,25,26,27,13]. However, this study used 43 different conditions to define multimorbidity, and compared only those testing positive compared to negative. The other UK Biobank study, between 16 March and 18 May, 2020 (1324 tested positive for COVID-19 and 426,875 remaining cohort), used Poisson regression analyses reporting that those with multimorbidity (defined using 43 conditions) had a 48% higher risk for a positive test for COVID-19 (RR 1.48 [95% CI 1.28, 1.71], P < 0.01). Although this study also reported that those with cardiometabolic multimorbidity, compared with those without, had a 77% higher risk for a positive test (RR 1.77 [1.46, 2.15] P < 0.01) [15], the outcome was based on testing positive in any setting (hospital or outpatient, pillar 1 and 2), which may introduce testing bias within UK Biobank. A recent article illustrated how the same dataset can produce apparently paradoxical findings for the same outcome [34]. For this reason, our study examined harder outcomes for those with severe SARS-CoV-2 infection; hospitalisation or death during the pandemic compared to the remaining cohort. Moreover, the three studies to date have only presented the number of conditions but have not examined the patterns of multimorbidity, i.e. the type of the two most common co-occurring conditions found in those with severe SARS-CoV-2 infection, nor the rate of severe infection or mortality in people with multimorbidity over the period of the pandemic.
Hypertension was found to be the most prevalent condition (40%) in those with severe SARS-CoV-2 infection, as in a number of previously published studies. Yet, when examined by clusters, we found that hypertension mainly co-existed with other conditions (i.e. diabetes, stroke, CKD). Thus, certain clusters of the multimorbidity index are more frequent than other clusters. When examining the association between the most common co-occurring multimorbidity clusters and the risk of severe SARS-CoV-2 infection, those with pre-existing CKD and diabetes showed the strongest association. For this reason, we believe that the classification of those with pre-existing multimorbidity index conditions is crucial to provide prognostic information to tailor effective treatment and prevent severe outcomes in common chronic conditions. Individuals with pre-existing multimorbidity conditions may have potential organ dysfunctions, which are further accelerated by the local and systemic effects related to SARS-CoV-2 infection. Previous studies have investigated the possible pathophysiological mechanisms linking this infection to severe COVID-19 syndrome, including increased inflammation, decreased immune response, heart failure, renal failure, and potentially multi-system organ failure and death [35, 36]. Further research is however required to understand the underlying biological mechanisms involved between these multimorbidity clusters and the increased risk of severe COVID-19 and its long-term sequelae (i.e., “long COVID”), particularly the effects on the immune system and frailty.
Multimorbidity has been associated with premature mortality, lower quality of life, polypharmacy, frailty, and fragmented care [10]. However, the current pandemic has placed additional risks on individuals with multimorbidity. A global survey evaluating the impact of COVID-19 on routine care for chronic diseases during 31 March to 23 April 2020 found that most healthcare professionals (67%) identified moderate or severe effects on their patients due to changes in healthcare services since the outbreak; and 80% reported the mental health of their patients worsened during COVID-19 [37]. Moreover, many low and middle-income countries have overstretched healthcare systems and the pandemic has further overwhelmed them.
A key strength of this study is the overall large sample size which allowed us to investigate whether the associations were heterogeneous across clinically relevant effect modifiers. We employed a comprehensive list of effect modifiers, extending past research [4], and enabling a greater understanding of the association between multimorbidity and severe SARS-CoV-2 infection. Moreover, we performed a literature review to derive an evidence based COVID-19 relevant multimorbidity index which can be applied to other populations [5,6,7,8, 20,21,22,23,24,25,26,27,28,29]. This study has some limitations. Issues of the low response rate (~ 5%) and selection bias, such as slightly higher representation of participants from affluent groups may suggest that the UK Biobank sample is not well representative of the UK population, and have been discussed previously [38]. The characteristics we examined were recorded at recruitment, representing data collected in the past at study baseline, but we conducted additional sensitivity analyses using the follow-up data for 25‐hydroxyvitamin D levels and the last recorded air pollution (NO2) data, showing consistent results over time. In further sensitivity analyses, we additionally adjusted the model for the time in the study, and found the results remained consistent. Factors such as cardiorespiratory fitness or C-reactive protein could possibly be a consequence of poor health from multimorbidity instead of cause, for this reason we removed these from the model, and the results remained the same. The data on multimorbidity were appraised only at baseline but there may have been a change in the number of multimorbidity index conditions since recruitment which were not accounted for and may lead to under or overestimation of effect. We were unable to assess the severity of disease conditions but our new multimorbidity index definition included conditions that were most relevant to COVID-19. Another limitation is the lack of detail of clinical severity of those with severe SARS-CoV-2 infection: while we have accounted for hospitalisation or death during the pandemic, further details of adverse COVID-19 outcomes such as length of hospital stay, intensive care admission, or mechanical ventilation, could provide further information. Ten potential effect modifiers were selected [4], although there are other factors which could possibly modify the association between multimorbidity and risk of severe SARS-CoV-2 infection but were unaccounted for, such as household size, occupation, and understanding and behaviour for social distancing, shielding, wearing facemasks and hand hygiene. Finally, this was an observational study, which limits our ability to establish causality.
Conclusion
In conclusion, having a new multimorbidity COVID-19 index that may help identify subjects at higher risk of complication in clinical practice could be beneficial. Where stratifying the risk according to the presence of multimorbidity, and reinforcing initiatives and resources to reduce the risk in this vulnerable group of individuals should be considered as priorities in the current coronavirus pandemic.
Availability of data and materials
The data that support the findings of this study are available from UK Biobank project site, subject to registration and application process. Further details can be found at https://www.ukbiobank.ac.uk. Statistical codes for this study are available at GitHub yc244.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- eGFR:
-
Estimated Glomerular Filtration Rate
- IQR:
-
Interquartile range
- NHS:
-
National Health Service
- OR:
-
Odds ratio
- RR:
-
Relative risk
- UK:
-
United Kingdom
- WHO:
-
World Health Organization
- 25(OH)D:
-
25-Hydroxyvitamin D
References
Worldometer, Coronavirus Update (Live). https://www.worldometers.info/coronavirus/. Accessed 29 Sept 2021.
Jorden MARS, Villarino E, Hoferka S, Patel MT, Bemis K. Evidence for limited early spread of COVID-19 within the United States. MMWR Morb Mortal Wkly Rep. 2020;69(22):680.
Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19—studies needed. N Engl J Med. 2020;382(13):1194–6.
South Asian Health Foundation: COVID-19 in Black, Asian and Minority Ethnic populations: an evidence review and recommendations from the South Asian Health Foundation. 2020.
Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e0241265.
Grasselli GZA, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–81.
Petrilli CM JS, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. 2020. https://doi.org/10.1136/bmj.m1966.
Safiya Richardson JSH, Narasimhan M, Crawford JM, McGinn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9.
Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo C-L, Kuchel GA, Melzer D. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort. J Gerontol Ser A. 2020;75(11):2224–30.
Academy of Medical Sciences, Multimorbidity: a priority for global health research. 2018. https://acmedsci.ac.uk/file-download/82222577. Accessed 2 Aug 2021.
Chudasama YVGC, Appiah K, Zaccardi F, Razieh C, Davies MJ, Yates T, Khunti K. Multimorbidity and SARS-CoV-2 infection in UK Biobank | Elsevier Enhanced Reader. Diabetes Metab Syndr. 2020;14(5):775–6.
Sur Roy A, Joshi A. Response to “Multimorbidity and SARS-CoV-2 infection in UK biobank.” Diabetes Metab Syndr. 2020;14(5):969–969.
Woolford SJ, D’Angelo S, Curtis EM, Parsons CM, Ward KA, Dennison EM, Patel HP, Cooper C, Harvey NC. COVID-19 and associations with frailty and multimorbidity: a prospective analysis of UK Biobank participants. Aging Clin Exp Res. 2020;32(9):1897–905.
Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M, Investigators S-R. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension. 2020;76(2):366–72.
McQueenie R, Foster HME, Jani BD, Katikireddi SV, Sattar N, Pell JP, Ho FK, Niedzwiedz CL, Hastie CE, Anderson J, et al. Multimorbidity, polypharmacy, and COVID-19 infection within the UK Biobank cohort. PLoS ONE. 2020;15(8):e0238091.
Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, Seidu S, Zaccardi F, Davies MJ, Khunti K. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: a systematic review and meta-analysis. Diabetes Obes Metab. 2020. https://doi.org/10.1111/dom.14124.
UK Biobank. UK Biobank: protocol for a large-scale prospective epidemiological resource. https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf. Accessed 2 Aug 2021.
UK Biobank. UK Biobank ethics and governance framework. https://www.ukbiobank.ac.uk/media/0xsbmfmw/egf.pdf. Accessed 2 Aug 2021.
Armstrong J RJ, Allen N, Crook D, Wilson D, Wyllie D, O'Connell AM. Dynamic linkage of COVID-19 test results between Public Health England’s Second Generation Surveillance System and UK Biobank. Microb Genom. 2020;6(7):mgen000397. https://doi.org/10.1099/mgen.0.000397.
Arentz MYE, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–4.
Du R-H, Liang L-R, Yang C-Q, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. https://doi.org/10.1183/13993003.00524-2020.
Guan W-j, Liang W-h, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Res J 2020;55(5):2000547. https://doi.org/10.1183/13993003.00547-2020.
Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, Na Y, Choi H, Gong SY, Choi YH, Ko KP, Im JS, Jung J. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020;35(25):e237. https://doi.org/10.3346/jkms.2020.35.e237.
Li XXS, Yu M, Wang K, Tao Y, Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan | Elsevier Enhanced Reader. J Allergy Clin Immunol. 2020;146(1):110–8.
Myers LC, The Permanente Medical Group KPNC, Oakland, Parodi SM, The Permanente Medical Group KPNC, Oakland, Escobar GJ, The Permanente Medical Group KPNC, Oakland, Liu VX, The Permanente Medical Group KPNC, Oakland. Characteristics of hospitalized adults with COVID-19 in an Integrated Health Care System in California. JAMA. 2020;323(21):2195–8.
Yao Q, Wang P, Wang X, Qie G, Meng M, Tong X, Bai X, Ding M, Liu W, Liu K, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130(5):390–9.
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5.
Yang X, Yu Y, Xu J, Shu H, **a J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. https://doi.org/10.1016/S2213-2600(20)30079-5.
Zhou FYT, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–62.
World Health Organisation. Ambient (outdoor) air pollution. 2018. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health. Accessed 2 Aug 2021.
British Nutrition Foundation, New advice on Vitamin. 2018. https://www.nutrition.org.uk/nutritioninthenews/new-reports/983-newvitamind.html?__cf_chl_jschl_tk__=ed44f3f38c4328aae4a0590dbbd1f0459a24ca07-1597345628-0-AX6jK_qtRoVBhgd7ZoVrGgsVW4bYr24Hv-6wRgtx1lu-l7yh6l2eMj6ls-QWmD4jwP8rxhmclDmuAKOAPdMQqew3D1K-yuzxVqMBQI_DocJ0t_UkLzbJFO9IJ2gaZXZ73z2avKzbMA4KGi-3aWC2pOQN3RbwjFmzL8CjU8m_wg6SRlj5kvV8hzaXV279BKKC-sqUBCwszXluGEc-xwpvWsEom0Fz-BMpi3vz1mVG5bD1CnZPxfriVX8lu_sXBuSXcXodKZcimdj53JorwnY8u1OPRH0ByABSWF0Nm1qLoNO6El3RP10C_7Llu3z0i7U97A5tQzCmCHfGgGDKTFK9RRCDDmTnr9l8mhYgGvE9n9rKwgkz2zpOD3EYIBCjACJS3utyuXV9OPHDgDQVMtuIJ7w. Accessed 2 Aug 2021.
Yates T, Zaccardi F, Dhalwani NN, Davies MJ, Bakrania K, Celis-Morales CA, Gill JMR, Franks PW, Khunti K. Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur Heart J. 2017;38(43):3232–40.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease. Circulation. 2003;107(3):499–511.
Yates T, Zaccardi F, Razieh C, Gillies CL, Rowlands A, Kloecker DE, Chudasama YV, Davies MJ, Khunti K. Framework to aid analysis and interpretation of ongoing COVID-19 research. Wellcome Open Res. 2020;5(208):208.
Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45(8):100618.
Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, Abosalif KOA, Ahmed Z, Younas S. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833–9.
Chudasama YV, Gillies CL, Zaccardi F, Coles B, Davies MJ, Seidu S, Khunti K. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020;14(5):965–7.
Swanson JM. The UK Biobank and selection bias. The Lancet. 2012;380(9837):110.
Acknowledgements
This research has been conducted using the UK Biobank Resource (Reference 36371). We acknowledge the support from the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM), and the NIHR Leicester Biomedical Research Centre. NGF acknowledges funding from the MRC Epidemiology Unit core support (MC_UU_12015/5), and NIHR Biomedical Research Centre Cambridge: Nutrition, Diet, and Lifestyle Research Theme (IS-BRC-1215-20014). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Funding
This research was funded by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM), and the NIHR Leicester Biomedical Research Centre, and a grant from the UKRI-DHSC COVID-19 Rapid Response Rolling Call (MR/V020536/1). NGF acknowledges funding from the MRC Epidemiology Unit core support (MC_UU_12015/5), and NIHR Biomedical Research Centre Cambridge: Nutrition, Diet, and Lifestyle Research Theme (IS-BRC-1215-20014).The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Concept and Design: NF, KK, YC. Statistical analysis: YC. Statistical support: NF, FZ, CG. Acquisition, analysis, or interpretation of data: NF, KK, FZ, CG, CR, TY, DK, AR, MD, NI, SS. Drafting of the manuscript: YC. Critical revision of the manuscript for important intellectual content: NF, KK, FZ, CG, CR, TY, DK, AR, MD, NI, SS. All authors agree to publish the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants gave written informed consent prior data collection. UK Biobank has full ethical approval from the NHS National Research Ethics Service (16/NW/0274).
Consent for publication
Not applicable.
Competing interests
KK is chair for SAGE subgroup on ethnicity and COVID-19 and an independent member of SAGE.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Flow chart of participants included in the UK Biobank Study. Table S1. Literature search on the most common pre-existing comorbidities in patients with severe SARS-CoV-2 infection. Table S2. Association between multimorbidity index using 3 or more conditions and risk of severe SARS-CoV-2 infection. Table S3. Sensitivity analyses using vitamin D levels at follow-up and last recorded air pollution levels. Table S4. Sensitivity analyses considering time in the study and removing cardiorespiratory fitness and C-reactive protein from the model. Checklist S1. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chudasama, Y.V., Zaccardi, F., Gillies, C.L. et al. Patterns of multimorbidity and risk of severe SARS-CoV-2 infection: an observational study in the U.K.. BMC Infect Dis 21, 908 (2021). https://doi.org/10.1186/s12879-021-06600-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06600-y