Abstract
Background
Platelet aggregation inhibitors (PAI) are among the most frequently prescribed drugs in older people, though evidence about risks and benefits of their use in older adults is scarce. The objectives of this systematic review are firstly to identify the risks and benefits of their use in the prevention and treatment of vascular events in older adults, and secondly to develop recommendations on discontinuing PAI in this population if risks outweigh benefits.
Methods
Staged systematic review consisting of three searches. Searches 1 and 2 identified systematic reviews and meta-analyses. Search 3 included controlled intervention and observational studies from review-articles not included in searches 1 and 2. All articles were assessed by two independent reviewers regarding the type of study, age of participants, type of intervention, and clinically relevant outcomes. After data extraction and quality appraisal we developed recommendations to stop the prescribing of specific drugs in older adults following the Grading of Recommendations Assessment Development and Evaluation (GRADE) methodology.
Results
Overall, 2385 records were screened leading to an inclusion of 35 articles reporting on 22 systematic reviews and meta-analyses, 11 randomised controlled trials, and two observational studies. Mean ages ranged from 57.0 to 84.6 years. Ten studies included a subgroup analysis by age. Overall, based on the evaluated evidence, three recommendations were formulated. First, the use of acetylsalicylic acid (ASA) for primary prevention of cardiovascular disease (CVD) in older people cannot be recommended due to an uncertainty in the risk-benefit ratio (weak recommendation; low quality of evidence). Secondly, the combination of ASA and clopidogrel in patients without specific indications should be avoided (strong recommendation; moderate quality of evidence). Lastly, to improve the effectiveness and reduce the risks of stroke prevention therapy in older people with atrial fibrillation (AF) and a CHA2DS2-VASc score of ≥ 2, the use of ASA for the primary prevention of stroke should be discontinued in preference for the use of oral anticoagulants (weak recommendation; low quality of evidence).
Conclusions
The use of ASA for the primary prevention of CVD and the combination therapy of ASA and clopidogrel for the secondary prevention of vascular events in older people may not be justified. The use of oral anticoagulants instead of ASA in older people with atrial fibrillation may be recommended. Further high quality studies with older adults are needed.
Similar content being viewed by others
Background
There is evidence that the use of multiple medications has been rising over the past years, especially among older people [1]. Platelet aggregation inhibitors (PAI) constitute some of the most frequently prescribed drugs among people aged ≥65 [2, 3]. They are indicated in the prevention of cardiovascular disease, during and after myocardial infarction or acute coronary syndrome, during and after angioplasty and stenting, in the prevention of stroke and transient ischaemic attacks (TIA), and in the prevention of peripheral artery occlusive disease [4,5,6]. The pharmacological mechanism of action of PAI is the inhibition of thrombocyte activation and/or impeding aggregation. The treatment goal is preventing thrombotic complications [7]. However, an undesirable effect of this platelet inhibition is an increase in the risk of bleeding [8].
Despite the benefit of reducing cardiovascular events, several studies show that PAI are frequently associated with hospital admission due to adverse drug events [9,10,11,12]. Some of these adverse drug events could be avoided, for instance by an increased monitoring of the use of drugs and regular medication reviews [9,10,11]. In the case of acetylsalicylic acid (ASA), secondary prophylaxis with Helicobacter pylori eradication and proton pump inhibitors reduces the risk of gastrointestinal bleeding [9].
The use of PAI has been questioned in older people due to a higher risk of adverse events compared to younger, healthier adults [13, 14]. This higher risk is attributable to changes in pharmacokinetics and pharmacodynamics and a higher risk of drug interactions in older people [15].
Evidence regarding the risks and benefits of antiplatelet drugs in older people is scarce, as most studies focus on younger patients with fewer co-morbidities [16]. Existing guidelines usually do not adapt for old age and multimorbidity [17]. Hence, the balance between risks and benefits of PAI in the management of cerebrovascular disease, peripheral artery occlusive disease, and coronary disease in older adults with multimorbidity is not clear [12]. We therefore set out to systematically review the available evidence regarding the use of PAI in older and multimorbid people.
The objectives of this Systematic Review (SR) are
-
To identify the risks and benefits of the use of PAI in the treatment or prevention of cerebrovascular disease, peripheral artery occlusive disease, and coronary disease in older adults.
-
To develop recommendations which will enable physicians to stop the use of PAI in the treatment or prevention of cerebrovascular disease, peripheral artery occlusive disease, and coronary disease in older adults based on current best evidence.
The developed stop-recommendations will be incorporated in an electronic decision support tool for general practitioners within the EU-Project PRIMA-eDS (Polypharmacy in chronic diseases: Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support) [18].
Methods
A SR was performed in accordance with the methodology described earlier [19] following a specific study protocol (available from the authors upon request). We will report on the results narratively.
Search strategy
As described in the publication of our methodology [19], we employed a step-wise approach that consisted of four searches, of which the consecutive one was only conducted when the prior one did not lead to recent and high quality results. Search 1 was targeted at SR and meta-analyses (MA) in the Cochrane Database of Systematic Reviews (OVID interface, 2005 onwards) and Database of Abstracts or Reviews of Effects (DARE, OVID interface, 1991 onwards). Search 2 was also directed at SR and MA, but extended to MEDLINE (OVID interface, 1946 onwards), EMBASE (OVID interface, 1974 onwards), Health Technology Assessment (HTA, OVID interface 2001 onwards), and International Pharmaceutical Abstracts (IPA, OVID interface 1970 onwards). Search 3a was performed to find single studies (randomized controlled trials (RCT) and observational studies (OS) from SR and MA not included in searches 1 and 2 due to not meeting our inclusion criteria but containing eligible studies. Search 3b looked for RCT and OS in MEDLINE, EMBASE, HTA and IPA.
For this SR, searches 1 and 2 were performed in December 8th, 2015. The search string (see additional file 1) was developed with the help of a PICOS (population, intervention, comparison, outcomes and study design) framework. In search 3a, we identified eligible randomised controlled trials and OS from SR and MA, which themselves were not eligible for inclusion in our review (mainly because they were not focussed at older people). In parallel to the study selection of searches 1 and 2, we prepared a list of references to be checked in search 3a. Search 3b was considered as not being necessary because the SR and MA retrieved covered all eligible studies (see results) and we did not expect to find any additional eligible studies. In addition to database searches, all the references of the included studies were checked to obtain a comprehensive list of studies. Study protocols were collected to consider future updates of the SR. We also obtained articles from other sources (e.g. hand search).
Study selection
Two reviewers (AR, MM) independently screened titles and abstracts. When the abstracts seemed to meet the inclusion criteria, full texts were retrieved and assessed for inclusion. When needed, a third reviewer (ARG) was consulted to solve any disagreements. At the end of each search stage, the quality and completeness of the obtained studies were assessed and it was decided whether or not to proceed to the next stage of the search.
Inclusion and exclusion criteria
Articles were assessed for inclusion regarding the type of study, age of participants, type of intervention, and clinical relevance of the outcomes.
The following articles were excluded: editorials, opinion papers, case reports, case series, narrative reviews, letters, qualitative studies, and OS which do not provide information regarding our outcomes. Articles not focussing on patient relevant outcomes were also excluded.
Table 1 displays details of the inclusion and exclusion criteria.
Data extraction and quality appraisal
Data extraction and quality appraisal were performed using piloted forms. One reviewer did data extraction and quality appraisal and a second reviewer checked the forms for completeness and accuracy. A third reviewer was used in cases of disagreement. Four reviewers (AR, CS, MM, MK) participated at this stage of the SR. Data extracted included the specific drugs and dosages, study methods, time to follow-up, characteristics of the participants, outcomes and results. The quality of the included studies was assessed using specifically validated assessment tools for each type of study design: for SR and MA the AMSTAR appraisal tool [20, 21] and for clinical trials the Cochrane Collaboration’s tool for assessing risk of bias [22]. For observational studies a selection of questions from the critical appraisal skills programme (CASP) was used [23, 24].
Development of recommendations
A document containing a summary of all included studies, emphasising the risks and benefits of PAI was developed. This document and the quality of the study provided the basis for the development of recommendations on the discontinuation of PAI in older adults with cerebrovascular disease, peripheral artery occlusive disease, and coronary disease. Recommendations were judged regarding strength and quality of the evidence using the Grading of Recommendations Assessment Development and Evaluation (GRADE) methodology [25,26,27]. The final recommendations were worded following a standardised scheme clarifying strength and quality. Four reviewers (ARG, AS, IK, MM) were involved in the development and approval of the recommendations.
Results
Literature search and inclusion of studies
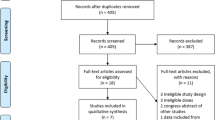
Figure 1 displays the identification process of studies for inclusion in the SR in a PRISMA flow-chart. Searches 1, 2 and 3a were performed. The research team decided not to perform search 3b for the reasons described above.
There were 964 references identified in the electronic databases during search 1 and 2. After the exclusion of all duplicates, a total of 853 references remained. Through other sources 1532 additional records were identified leading to a total number of 2385 screened records.
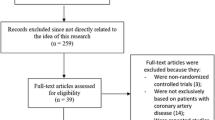
Out of those, 403 were identified and selected for full text evaluation, which led to the exclusion of 368 studies. Only 35 articles published between 1987 and 2016 met all inclusion criteria. A list of excluded studies along with the reason for exclusion is available from the authors upon request. The most frequent reason for exclusion was not meeting our age group target.
Among the included studies, there were 22 SR and MA, 11 RCT, and 2 OS. An overview of the main characteristics and quality of the included studies is presented in Tables 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11. PAI were tested for the following indications: ASA in primary [28,29,30,31,32,33,34,35] and secondary prevention of cardiovascular disease (CVD) [36], ASA in the primary and secondary prevention of stroke in patients with and without AF [30, 34, 37,38,39,40,41,42,43,44,45,46,47,48,49,50]ADP-receptor inhibitors in secondary prevention of cardiovascular events [51, 52] and stroke/TIA [52,53,54], and dipyridamol in secondary prevention of stroke [55, 56]. Regarding the demographics of the sample, the mean age of participants in the included studies ranges between 57 to 84.6 years. This wide range is due to ten [28, 29, 31, 33, 36, 51, 52, 56, 57] studies that were included because of a subgroup analysis of people aged above 65 years despite a mean age below the threshold. Polypharmacy was not assessed in any of our included studies. None of the included papers reported on the outcomes quality of life, hospitalisation and life expectancy. Information on the presence of comorbidities was reported by 26 of the included studies, mostly consisting of the presence of cardiovascular risk factors or cardiovascular diseases such as stroke and TIA. Coincident medications were declared in 7 of 40 included references. Frailty was only reported by one study [58] and cognitive status by none (see Tables 9, 10 and 11).
Effectiveness and safety of PAI
ASA in the primary prevention of CVD
Three SR/MA [28,29,30] and five RCT [31,32,33,34,35] were included, which examined the primary prevention of CVD. Tha MA of Baigent et al. [28] detected an insignificant reduction in the occurrence of a composite endpoint of serious vascular events including myocardial infarction, stroke, or death from a vascular cause (including sudden death, pulmonary embolism, haemorrhage) in the subgroup of participants older than 65 years (RR 0.88; 95% CI: 0.77–1.01). Even in the complete study group, the benefit of ASA with an absolute risk reduction (ARR) of 0.06% per year for serious vascular events was very low (number needed to treat (NNT) = 1666 per year). Moreover, there was no difference in vascular or all cause mortality between the ASA group and the placebo group (0.19% vs. 0.19% per year; p = 0.7), whereas the risk of major gastrointestinal and extracranial bleeds increased under a treatment with ASA (0.10% vs. 0.07% per year, p ≤ 0.0001) (secondary endpoints and adverse effects not calculated for older subgroup).
The study of Kjeldsen et al. (2000) with 18,790 participants [31] revealed a significant relative risk reduction in the occurrence of myocardial infarction in the subgroup of participants ≥65 years (RR 0.62; 95% CI: 0.38–0.98; p = 0.04), but the relative risk of major cardiovascular events was not significantly reduced in the age group ≥65 years (RR 0.92; 95% CI: 0.74–1.15; p = 0.47).
The clinical trial of Ikeda et al. (2014) [35] including 14,464 adults with a mean age of 70 years analysed the impact of ASA on the risk of cardiovascular events in older Japanese patients with multiple atherosclerotic risk factors in comparison to placebo. The primary endpoint of this study was a composite of death from cardiovascular causes (myocardial infarction, stroke, and other cardiovascular causes), nonfatal stroke (ischemic or haemorrhagic), and nonfatal myocardial infarction. Overall, no significant difference in the occurrence of the composite endpoint was observed between the two groups (hazard ratio (HR): 0.94; 95% CI: 0.77–1.15; p = 0.54). Moreover, in comparison to the placebo group, the treatment with ASA was associated with a significant increased risk of extracranial haemorrhage requiring transfusion or hospitalization (HR for ASA: 1.85 (95% CI: 1.22–2.81); p = 0.004), absolute risk increase 0.35, number needed to harm 286).
Concerning the risk of bleeding in the primary prevention with ASA in older people, the RCT of Silagy et al. [32] with the oldest participants in this subject area (participants n = 400, mean age of participants 73 years) identified more gastrointestinal bleeding events in the ASA group in comparison to the placebo group (3% vs. 0%) with a significant decrease in mean hemoglobin levels (0.33 g/dl vs. 0.11 g/dl; p < 0.05). He et al. (1998) [29] conducted in a MA a subgroup-analysis of participants above and below 64 years with regard to the risk of haemorrhagic stroke with ASA in comparison to placebo. In the subgroup of participants ≥64 years, the absolute risk difference between the ASA and placebo group was 34 per 10,000 persons (95% CI: 1–66).
The only benefit for ASA was suggested in the RCT of Ogawa et al. (2008) with 2539 participants [33], (which included only people with diabetes mellitus), where in the subgroup of people older than 65 years the benefit in reducing atherosclerotic events (including fatal or nonfatal ischemic heart disease, fatal or nonfatal stroke, and peripheral arterial disease) was significant (HR 0.68; 95% CI: 0.46–0.99; p = 0.047).
ASA in the secondary prevention of CVD
The trial of Huyhn et al. (2001) with 135 participants [36], analysed the effectiveness of ASA, warfarin, and the combination therapy of ASA and warfarin in participants with prior bypass surgery for the secondary prevention of coronary events. The primary endpoint of this study was a composite of any-cause death, myocardial infarction, or unstable angina requiring a new hospitalization. Monotherapy with ASA as well as ASA plus warfarin were associated with the lowest event rate of the composite endpoint (14.6% warfarin, 11.5% ASA, 11.4% ASA warfarin, p = 0.76). For patients aged >65 years, an overall higher event rate was detected, but ASA monotherapy revealed the lowest event rate (41.7, 34.8% and 36.8 events respectively). HR were not reported in this publication.
ASA in the primary prevention of stroke
Patients with AF: Six SR/MA [38,39,40, 42, 44, 49] showed conflicting evidence regarding the benefit of ASA compared to placebo in the primary prevention of stroke and all cause mortality. While four of the SR demonstrated no benefit for ASA [38, 40, 44, 49] (OR for stroke 0.70 (95% CI:0.47–1.07) [49], 0.56 (95% CI:0.19–1.65) [38] and RR 0.81 (95% CI:0.65–1.01) [40] and 1.30 (95% CI:0.96–1.72) [44] respectively, and for mortality 0.75 (95% CI:0.54–1.04) [49], 0.87 (95% CI: 0.68–1.12) [38] and RR 0.86 (95% CI:0.69–1.07) [40] and 1.28 (95% CI:0.98–1.65) [44], respectively), two SR [39, 42] found a significant benefit for stroke, but not for mortality (RR of 0.64 (95% CI: 0.44–0.88) for stroke, no data for mortality [42], RR 0.78 (95% CI: 0.62–0.98) for stroke and 0.84 (95% CI:0.67–1.05) for mortality [39]).
In all included trials with ASA [38, 40, 42, 44, 49, 59] except for one MA [39], ASA increased the risk of bleeding, especially for gastrointestinal bleeding in comparison to placebo. In the SR of Coleman et al. (2012) [60], the risk of major gastrointestinal bleeding was three times greater under a treatment with ASA compared with placebo. However, these results did not reach statistical significance (odds ratio (OR) 3.23; 95% CI: 0.56–18.66). Connolly et al. (2013) [61] also showed an increased risk of a subdural haematoma under a treatment with ASA in comparison to placebo (OR 2.2; 95% CI: 0.6–7.8; p = 0.6), but this was not significant.
Eleven SR/MA [30, 37,38,39,40,41,42,43,44,45,46], one RCT [47] and one OS [48] reported that ASA was less effective in preventing stroke in patients with AF than warfarin. The risk of nonfatal ischemic stroke and systemic embolism was significantly higher with a treatment with ASA compared to warfarin [30, 37, 39, 41, 43, 45, 47, 62]. Apart from the results of the MA of Dogliotti et al. [41], there was no significant difference in mortality between the two groups [38,39,40, 44, 45, 47]. With the exception of six trials [30, 42, 45,46,47, 57], bleeding events were significantly less frequent in all included studies [37,38,39,40,41, 43, 44, 48, 60] when patients were treated with ASA compared to warfarin. Concerning the use of new oral anticoagulants (NOAC) in older people, ASA were associated with a higher risk of stroke or systemic embolism than NOACs in all included studies [41, 44, 46, 63]. The MA of Lin et al. (2015) [63] showed that in ≥75 years old people ASA was less beneficial concerning the prevention of stroke and systemic embolism compared to the dabigatran treated group (dabigatran 110 mg vs. ASA rate ratio: 1.31 (95% CI: 0.84–2.07). Concerning the risk of bleeding inconsistent results were detected. ASA was associated with a decreased risk of bleeding events in comparison to NOACs (apixaban vs. ASA: OR 0.88; 95% CI: 0.31–2.18 [41]; ASA vs. edoxaban: RR 2.41; 95% CI: 1.02–6.80) [44]. However, in the SR of Cameron et al. (2014) [46], ASA increased the risk of major bleeding events (ASA <100 mg/d vs. edoxaban 30 mg/d: OR 2.27; 95% CI: 1.26–4.1).
Patients without AF
The clinical trial of Uchiyama et al. [50] with 14,464 participants (mean age 70 years) analysed the impact of ASA on the risk of stroke and intracranial haemorrhage in older Japanese patients without AF in comparison to placebo. Overall, no significant difference in the occurrence of the cumulative rate of fatal or nonfatal stroke was observed between the two groups (HR: 0.92; 95% CI: 0.74–1.16; p = 0.51). Five years after randomization, the cumulative rate of fatal or nonfatal stroke in the ASA group was 2.068% (95% CI: 1.75–2.44) as opposed to 2.29% (95% CI: 1.96–2.69) in the placebo group (HR 0.927; 95% CI: 0.741–1.160; p = 0.509). Moreover, in comparison to the placebo group, a non-significant reduction of the risk of ischemic stroke or transient ischemic attack was observed in the ASA group (HR 0.783; 95% CI: 0.606–1.012; p = 0.061). A treatment with ASA, was associated with a non-significant increase in risk of intracranial haemorrhage in comparison to the placebo group (HR 1.46; 95% CI: 0.956–2.237; p = 0.078).
ASA in the secondary prevention of stroke
Patients with AF
In these patients, ASA in comparison to placebo showed a higher reduction in secondary than in primary prevention [39, 40]. The ARR of stroke was between 1.5% (NNT = 67) [39] and 0.8% per year (NNT= 125) [40] in the primary prevention trials, and 2.5% per year (NNT = 40) in the secondary prevention trials [39, 40]. In the EAFT trial with 1007 participants [34] no significant reduction in the risk of a recurrent stroke by ASA in comparison to placebo was observed (HR 0.86; 95% CI: 0.64–1.15), while the risk of bleeding non-significantly increased under the treatment with ASA (HR 1.3; 95% CI: 0.8–2.15). Warfarin was much more effective than ASA in the secondary prevention of stroke leading to a significant relative risk reduction of 40% in the occurrence of a recurrent stroke (HR 0.60; 95% CI: 0.41–0.87; p = 0.008) [34]. On the other hand, the risk of bleeding was 2.8 fold higher in the warfarin group than in the ASA group (HR 2.8; 95% CI: 1.7–4.8; p < 0.001) [34].
Patients without AF
One study [58] including 505 patients with cerebral infarction, minor or major stroke and a mean age of 68 years analysed the secondary prevention of stroke with ASA in comparison to placebo. The primary endpoints of this study were the recurrence of stroke and death. The incidence of stroke recurrence was 6.3% in the ASA treated group and 6.4% in those randomised to placebo. The OR for stroke recurrence and death comparing ASA to placebo was 1.04 (95% CI: 0.68–1.58), reflecting no significant difference between both groups.
ADP-receptor inhibitors in the secondary prevention of CVD
One RCT including 13,608 adults with a mean age of 61 years and a subgroup analysis with people older than 75 years, compared prasugrel and clopidogrel for the management of acute coronary syndromes with scheduled percutaneous coronary intervention [51]. The primary endpoint of this study was a composite of cardiovascular mortality, non-fatal myocardial infarction, or non-fatal stroke. In all included patients, the composite primary endpoint (as mentioned above) was reached in 12.1% of patients randomised to clopidogrel and 9.9% of those randomised to prasugrel (HR 0.81; 95% CI: 0.73–0.90; p ≤ 0.001). Moreover, prasugrel was more effective in reducing the rates of myocardial infarction (9.7% for clopidogrel vs. 7.4% for prasugrel; p ≤ 0.001), urgent target-vessel revascularization (3.7% vs. 2.5%; p ≤ 0.001), and stent thrombosis (2.4% vs. 1.1%; p ≤ 0.001). Several subgroup-analyses were carried out. One subgroup-analysis of participants aged ≥75 years considered the composite endpoint of death from any cause, nonfatal myocardial infarction, nonfatal stroke, or non-CABG-related nonfatal major bleeding. It showed that in the subgroup of patients older than 75 years, there was no benefit of prasugrel in comparison to clopidogrel regarding this composite endpoint (HR 0.99; 95% CI: 0.81–1.21; p = 0.92). Another subgroup-analysis examined the combined endpoint of death from any cause, nonfatal myocardial infarction, and nonfatal stroke under a treatment with prasugrel or clopidogrel in three different age groups (<65 years, 65–74 years, and ≥75 years). In the age group of patients <65 the combined endpoint (as mentioned above) was reached in 8.1% in the prasugrel group compared to 10.6% in the clopidogrel group (risk reduction 25%, HR not reported). In the age group between 65 years and 74 years the occurrence of the combined endpoint was 10.7% in the prasugrel group and 12.3% in the clopidogrel group (risk reduction 14%, no HR reported). In the age group of participants ≥75 years, the risk reduction attributed to prasugrel in comparison to clopidogrel was the lowest of the considered three age groups (17.2% prasugrel group, 18.3% clopidogrel group, risk reduction of 6%, HR or OR not reported). The MA of Zhou et al. [52] with 7 trials including 48,248 participants, investigated the risks and benefits of a dual therapy with ASA and clopidogrel vs. monotherapy for the secondary prevention of cardiovascular and cerebrovascular events (see below). The population, included in this MA, were a mixed population. The participants had for example atrial fibrillation, multiple atherothrombotic risk factors, previous coronary artery bypass grafting/PCI or acute coronary syndromes without ST-segment elevation. The combination therapy was non-significantly more effective than the single drug therapy alone in reducing the rate of major cardiovascular events (9% RR reduction; 95% CI: 2–17) when all participants were included. The relative risk of MI was decreased by 14% (RRR 14%; 95% CI: 3–24). Overall, the ARR of major cardiovascular events due to the combination therapy was 1.06 with a NNT of 83. On the other hand, the combination therapy resulted in a significant 62% RR increase of major bleeding events (95% CI: 26–108) when compared to single drug therapy. For the subgroup analysis of participants older than 65 years, a comparison between the combination therapy and a monotherapy with ASA was performed. In the older participants (≥65 years), the reduction of major cardiovascular events was marginally significant (≥65 years RR: 0.90; 95% CI: 0.83–0.98), whereas the risk of major bleeding events under a treatment with ASA plus clopidogrel vs. ASA monotherapy was significantly higher (≥65 years: RR: 1.56; 95% CI: 1.29–1.89).
ADP- receptor inhibitors in the secondary prevention of stroke and/or transient ischemic attack
The MA of Zhou et al. [52] described above also investigated, the secondary prevention of cardiovascular events, and the secondary prevention of stroke. With regard to this outcome, the greatest reduction was detected in the occurrence of stroke (RR 16%; 95% CI: 1–28).
In the RCT of Diener et al. (2004) with 7599 participants [53] the benefit to risk ratio did not show the additional clinical value of adding ASA to clopidogrel in high-risk patients with transient ischaemic attack or ischaemic stroke. A subgroup analysis (n = 4537) by age (≥65 years) showed that the event rate for clopridogrel plus ASA was 17.4% and for clopidogrel plus placebo 17.7%.
The dual antiplatelet therapy (DAPT) with ASA and clopidogrel was associated with an increased risk of 30-day major stroke, spontaneous MI, all-cause mortality, and combined lethal and major bleeding in the DAPT group compared to monotherapy even in patients who underwent Transcatheter Aortic Valve Implantation (TAVI) (OR 1.88; 95% CI: 1.00–3.56). The biggest increase was detected in the occurrence of lethal and major bleeding events (OR 2.62; 95% CI: 1.29–5.33) [54].
Dipyridamol (DP) in the secondary prevention of stroke
The MA of Leonardi-Bee et al. [55] with 11,459 participants including a subgroup-analysis of participants older than 65 years identified a non-significant decrease in the reoccurrence of stroke under treatment with DP in comparison to placebo (OR 0.82; 95% CI: 0.68–1.00). In the subgroup of participants ≥65 years the reduction of stroke was non-significant (DP vs. placebo subgroup ≥65 years: OR 0.81; 95% CI: 0.65–1.02). The combination therapy of ASA + DP in comparison to an ASA monotherapy revealed a significant reduction of stroke [55, 56] (ASA + DP vs. ASA monotherapy: Age ≥ 65 years: OR 0.78; 95% CI: 0.63–0.97) [55]. There was no difference in mortality between the two treatment groups (ASA + DP vs. ASA: HR 1.01, 95% CI 0.87–1.17) [56].
Quality appraisal of included studies
SR and MA
Table 5 displays the results of quality appraisal of the SR and MA. One MA [52] fulfilled all requirements of the AMSTAR appraisal tool. Several quality deficits were detected when evaluating the other studies using the AMSTAR appraisal tool. In all included MA/SR an a priori design was provided. A duplicate study selection and data extraction were missing in the MA/SR of Baigent et al. [28], Lip et al. [45] and Cooper et al. [42]. In the SR/MA of Leonardi-Bee et al. [55], Taylor et al. [37], and Assiri et al. [44] this information was not available. A comprehensive literature search was not performed in the MA/SR of Connolly et al. [61] and He et al. [29]. Eleven MA [28, 29, 38, 40,41,42,43,44,45, 56, 60] did not search for grey literature. Quality appraisal of the included studies was not performed in 10 MA/SR [28, 29, 39, 41,42,43, 45, 46, 56, 61]. Possible conflicts of interest were not declared in four MA [39, 42, 60, 61]. All included SR/MA described the characteristics of the included studies. The likelihood of publication bias was presented in seven MA/SR [37, 43, 45, 52, 54, 60, 63].
RCTs
Table 6 displays the results of quality appraisal of the RCTs. An appropriate random sequence generation was used in seven [31, 33,34,35, 50, 53, 58] of 11 RCTs. In four RCTs [32, 36, 47, 51], the random sequence was unclear. Allocation concealment was fulfilled in six studies [33,34,35, 50, 53, 58] and unclear in five studies [31, 32, 36, 47, 51]. Serious limitations were found in blinding of personnel and participants in five RCTs [33,34,35, 47, 50], whereas Huynh et al. [36] and Britton et al. [58] performed appropriate blinding of personnel and participants. In four RCTs [31, 32, 51, 53] this remained unclear. The outcomes were unlikely to be influenced by a lack of blinding in five studies [33,34,35, 50, 58]. The blinding of trials was appropriate in five studies [33,34,35, 50, 58]. In the remaining studies the blinding outcome was unclear. In the study of Ogawa et al. [33], a high risk for selective reporting was detected due to a missing representation of adverse events in the subgroup analysis of adults ≥65 years of age. Inclusion and exclusion criteria for participants and the primary outcomes were clearly defined and stated in all studies. In two trials [31, 36] differences between the treatment groups after randomisation were identified. The loss to follow-up was less than 5% in six trials [31, 32, 36, 51, 53, 58] whereas the RCT of Ogawa et al. [33], the EAFT trial [34] and the clinical trial of Ikeda et al. [35] had a higher loss to follow-up. In the two other trials [47, 50] it remained unclear. Conflicts of interests were stated in all RCTs except in two trials [31, 34]. In the EAFT [34] trial a high risk for biased selection of participants was detected because all participants who were not eligible for a treatment with warfarin (e.g. due to previous bleeding events) were assigned to the ASA group.
Observational studies
Table 7 displays the results of quality appraisal of the OS. In the two included OS [48, 59] we could not identify important confounding factors due to a lack of information and an undersized database. The results could be influenced by a lack of blinding of personnel and participants in all included studies.
Development of recommendations
We developed three recommendations which are presented in Table 8. One recommendation was rated as strong with moderate quality of evidence. The two other recommendations were assessed as weak with low quality of evidence. Table 8 reports on the main articles, which constitute the evidence base for each recommendation, although all included studies were taken into account for the risk/benefit balance during the review process. The quality appraisal of each RCT included in the SR and MA provided the evidence base for the recommendations. All quality appraisals were considered in assessing the quality of the evidence of the recommendations and are available from the authors upon request. Based on the evaluated evidence we formulated three recommendations. The first recommendation deals with the use of ASA in the primary prevention of CVD and stroke in older people without diabetes. The strength of the recommendation is weak and the quality of the evidence was judged as low. Concerning the primary prevention of CVD and stroke in the elderly with ASA, no benefit could be shown in patients without AF compared to placebo. Moreover, the risk of haemorrhagic stroke [28, 29], major gastrointestinal [28, 32] and other extracranial non-fatal bleeds [28, 31, 35] were significantly increased. In contrast, for people with diabetes mellitus the trial of Ogawa et al. [33] showed that the greatest benefit of a treatment with ASA in comparison to placebo was detected in the subgroup of participants older than 65 years. Due to this effect, adults with diabetes mellitus were not included in our recommendation. However, a high risk for selective reporting was detected in this study due to a lack of reporting adverse events in the subgroup analysis of adults ≥65 years of age.
Overall, we reached similar conclusions to the Beers criteria for potentially inappropriate medications in older people, which recommend using ASA with caution in adults older than 80 years for primary prevention of CVD [64, 65].
The second recommendation was developed based on the evidence of MA of Zhou et al. [52] and the RCT of Diener et al. [53]. We recommend avoiding the combination of a dual therapy in the secondary prevention of TIA and stroke with clopidogrel and ASA and to consider monotherapy instead. The evidence shows that a dual therapy increases the risks of bleeding complications and is not beneficial in the secondary prevention of vascular events, especially in the subgroup of adults aged 65 years or older. Adults with another indication for dual therapy (see Table 8) must be excluded from this recommendation. Due to the high quality of the evidence base the strength of the recommendation was rated as strong whereas the quality of evidence was downgraded to moderate quality caused by the indirectness of results. This recommendation was similar to the recommendation of the STOPP/START criteria for potentially inappropriate prescribing in older people, which recommends to stop a dual therapy with ASA and clopidogrel for secondary prevention of stroke (expections are: the patient underwent coronary stenting in the previous 12 months or has a high grade symptomatic carotid arterial stenosis) [66].
The third recommendation is to discontinue the use of ASA for the primary prevention of stroke in older adults with AF (including adults older than 75 years), because current evidence points at an unfavourable risk/benefit ratio for ASA compared to placebo. None of the identified SR demonstrated a benefit regarding mortality, and only two older SR [39, 42] appear to show a benefit regarding stroke. The most recent and reliable SR including a comprehensive network meta-analysis does not show this benefit [44]. Instead, the use of a Vitamin K Antagonist should be considered. This recommendation is based on the evidence of eleven SR/MA [30, 37,38,39,40,41, 46, 57, 63] and one clinical trial [47]. The recommendation was rated as weak and the quality of the evidence as low. The evidence showed a superiority of warfarin in the prevention of cerebrovascular diseases. In regard to the risk of bleeding, contrasting results were found. With the exception of three trials [46, 47, 57], bleeding events were significantly more frequent when compared to ASA. However, the trial [47] with the oldest participants suggested a benefit of warfarin over ASA in octogenarians. There were significantly more ischemic strokes and systemic embolism with ASA than with warfarin but there were significantly fewer adverse events (including bleeding) with warfarin than ASA, assuming a safe handling even in adults older than 80 years. The dose of ASA and the target-INR in the included studies were roughly comparable. A major limitation of this recommendation is that the dose of ASA of 300 mg per day (as it was used in several studies) was higher than the usual applied dose.
We were limited to providing three recommendations to stop treatments because of a lack of reliable studies in our age group. In relation to secondary prevention, the recommendations for the use of PAI are mainly based on studies of younger patients, and it is currently unknown whether these recommendations are transferable to older people. Nonetheless, taking current best evidence regarding younger patients into account, it does not seem justified to formulate a stop recommendation for older people.
Discussion
Our systematic review examined the benefits and risks of the treatment with PAI for the management of cardiovascular, cerebrovascular and peripheral vascular diseases in older people. This systematic review is part of a compilation of systematic reviews on commonly used drugs in older people and aimed to identify the evidence to develop recommendations on when to discontinue the inappropriate use of these medications in older adults. Based on the evaluated evidence we formulated three recommendations.
Our SR has strengths as well as limitations. To the best of our knowledge, this is the first SR that has searched the evidence on the use of PAI specifically amongst older people. We followed a standard methodology as recommended by the Cochrane collaboration and the PRISMA statement, used a predefined step-wise search approach and piloted our search strategy. This systematic search strategy has the advantage that the search strategy is transparent and reproducible and will have utility in assessing the evidence for treatments aimed specifically at older people. Unfortunately, many papers had to be excluded as they did not report on the evidence for treatments in older people reflecting the lack of studies in this age group. An example was the lack of evidence concerning the secondary prevention of stroke and cardiovascular disease with ASA in comparison to placebo. The only study for secondary prevention of CVD, that met our inclusion criteria, was a small study with 132 participants and insignificant results. Current guidelines on recommendations to prescribe ASA in the secondary prevention of CVD and stroke are therefore based on study evidence derived from younger patients [67]. We do not know whether the benefits shown in these studies are also applicable to older people. There are ongoing studies targeted at filling this evidence gap. The largest of these studies is the ASPREE Trial (study protocol published 2013) [68], taking place in Australia. It included 16,700 participants aged 70 years and older and aims to analyse the impact of daily low-dose aspirin on cardiovascular disease (heart attack and stroke) in older people. The results of the ASPREE trial are expected to be published in 2018.
Another limitation is that our search strategy resulted in SR and MA with overlap** studies (see additional file 2). Altogether, 143 studies were included in the SR and MA and out of these studies, 40 studies were counted repeatedly. This probably meant that outcomes from these overlap** studies would be weighted more positively in our analyses compared to studies, which have only been included once. Despite the overlap, we decided to include all SR and MA because they offered additional relevant information.
We included two different types of MA namely standard MA based on head-to-head comparisons and network MA making indirect comparisons. Although the strength of evidence of network MA in general is considered to be weaker than that of standard MA, this would not have led a different conclusion in our SR. It is important to note that due to our methodology we could not take into account the strength of evidence of network MA which is generally weaker.
During the development of our recommendations we weighted the benefits and risks for using platelet aggregation inhibitors in older people [19]. We did not assess the material for people of other ages and we did not look for possible start recommendations because our study had the specific aim of hel** to reduce polypharmacy in older people. The widespread use of ASA contributes significantly to the problem of polypharmacy. [69]. With the implementation of our recommendations we hope to contribute to a reduction in the treatment with PAI and hence reduce inappropriate polypharmacy. We hope our recommendations will lead to the development of new guidelines specifically addressing the drug treatment of old and multi-morbid adults. We are currently using these recommendations in an electronic decision support tool aimed at reduce polypharmacy in a multicenter, randomised, controlled PRIMA-eDS trial with 3900 patients [18].
Conclusions
Based on the evaluated evidence, this systematic review was able to develop three recommendations. The use of ASA for the primary prevention of CVD and the combination therapy of ASA and clopidogrel for the secondary prevention of vascular events in older people may not be justified when the risk-benefit ratio is taken into account. The use of warfarin instead of ASA in older patients with AF may be recommended. To improve the effectiveness and reduce the risks of stroke prevention therapy in older people with AF, the discontinuation of ASA for the primary prevention of stroke should be considered and oral anticoagulants could be used instead (low quality of evidence).
Older patients with multimorbidity and polypharmacy are underrepresented in clinical trials. None of the articles, that we identified, reported on patients with polypharmacy. We were therefore not able to develop recommendations for reducing polypharmacy in patients who are old and with multi-morbidity. The benefits of many treatments for these patient groups are less clear and further good quality studies are needed for example RCTs investigating the individualised assessment of multi-morbid people with polypharmacy (including PAI).
We expect our recommendations in addition with the other recommendations of the PRIMA-eDS trial to contribute to the development of new guidelines specifically addressing the drug treatment of old adults with multi-morbidity.
Abbreviations
- AF:
-
Atrial fibrillation
- AMSTAR:
-
Assessment of multiple systematic reviews
- ARR:
-
Absolute risk reduction
- ASA:
-
Acetylsalicylic acid
- CASP:
-
Critical Appraisal Skills Programme
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- DAPT:
-
Dual- antiplatelet therapy
- DARE:
-
Database of Abstracts or Reviews of Effects
- DP:
-
Dipyridamole
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- HR:
-
Hazard ratio
- HTA:
-
Health Technology Assessment
- IPA:
-
International Pharmaceutical Abstracts
- MA:
-
Meta-analysis
- NNT:
-
Number needed to treat
- OR:
-
Odds ratio
- OS:
-
Observational study
- PAI:
-
Platelet aggregation inhibitors
- PICOS:
-
Population, intervention, comparison, outcomes and study design
- PRIMA-eDS:
-
Polypharmacy in chronic diseases: Reduction of Inappropriate Medication and Adverse drug events in older populations by electronic Decision Support
- RCT:
-
Randomised controlled trial
- SR:
-
Systematic review
- TIA:
-
Transient ischaemic attack
- VKA:
-
Vitamin K Antagonist
References
Hovstadius B, et al. Increasing polypharmacy - an individual-based study of the Swedish population 2005-2008. BMC Clin Pharmacol. 2010;10:16.
Onder G, et al. Advanced age and medication prescription: More years, less medications? A nationwide report from the Italian medicines agency. J Am Med Director Assoc. 2016;17(2):168-72.
Flaherty JH, et al. Polypharmacy and hospitalization among older home care patients. J Gerontol A Biol Sci Med Sci. 2000;55(10):M554–9.
Gregg D, Goldschmidt-Clermont PJ. Platelets and cardiovascular disease. Circulation. 2003;108(13):e88–90.
Silber S. Evidence-based management of ST-segment elevation myocardial infarction (STEMI): latest guidelines of the European Society of Cardiology (ESC) 2010. [German] Evidenzbasiertes Vorgehen beim ST-Strecken-Hebungsinfarkt (STEMI): Neueste Leitlinien der Europaischen Gesellschaft fur Kardiologie (ESC) 2010. Herz. 2010;35(8):558–65.
Perk J, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). G Ital Cardiol (Rome). 2013;14(5):328–92.
Lüllmann H, Mohr K, Hein L. Pharmakologie und Toxikologie. Georg Thieme Verlag. 2010;207(9):314.
Patrono C, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373–83.
Howard RL, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63(2):136–47.
Kongkaew C, et al. Risk factors for hospital admissions associated with adverse drug events. Pharmacotherapy. 2013;33(8):827–37.
Davies EC, et al. Emergency re-admissions to hospital due to adverse drug reactions within 1 year of the index admission. Br J Clin Pharmacol. 2010;70(5):749–55.
Salvi F, et al. Adverse drug events as a cause of hospitalization in older adults. Drug Saf. 2012;35(1):29–45.
Fick DM, et al. Updating the beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–24.
Sorensen HT, et al. Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin. Am J Gastroenterol. 2000;95(9):2218–24.
Franchini M. Hemostasis and aging. Crit Rev Oncol Hematol. 2006;60(2):144–51.
Van Spall HG, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233–40.
Boyd CM, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24.
Sonnichsen A, et al. Polypharmacy in chronic diseases-reduction of inappropriate medication and adverse drug events in older populations by electronic decision support (PRIMA-eDS): study protocol for a randomized controlled trial. Trials. 2016;17(1):57.
Martinez YM, R.-G.A., Reeves D, Ediriweera de Silva RE, Esmail A, Kunnamo I, Rieckert A, Sommerauer C, Sönnichsen A, A set of systematic reviews to help reduce inappropriate prescribing to older people: study protocol. BMC Geriatrics. 2017;17(1).
Shea BJ, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
Shea BJ, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–20.
Higgins JPT, G.S., (editors). Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from http://handbook-5-1.cochrane.org/. 2011. Last accessed 18 Aug 2017.
Critical Appraisal Skills Programme. 11 questions to help you make sense of case control study. 2013; Available from: https://hhs.hud.ac.uk/lqsu/Useful/critap/Case%20Control%20Study%20Checklist/CASP-Case-Control-Study-Checklist-31.05.13.pdf. Accessed 18 Aug 2018.
Critical Appraisal Skills Programme. 12 questions to help you make sense of cohort study. 2013; Available from: https://hhs.hud.ac.uk/lqsu/Useful/critap/Cohort%20Study%20Checklist/CASPCohort-Study-Checklist-31.05.13.pdf. Accessed 18 Aug 2017.
Guyatt GH, et al. Going from evidence to recommendations. BMJ. 2008;336(7652):1049–51.
Guyatt GH, et al. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–8.
Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Baigent C, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–60.
He J, et al. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA. 1998;280(22):1930–5.
Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;(3):Cd006186.
Kjeldsen SE, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension optimal treatment. J Hypertens. 2000;18(5):629–42.
Silagy CA, et al. Adverse effects of low-dose aspirin in a healthy elderly population. Clin Pharmacol Ther. 1993;54(1):84–9.
Ogawa H, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300(18):2134–41.
Group, E.A.F.T.S. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial fibrillation trial) study group. Lancet. 1993;342(8882):1255–62.
Ikeda Y, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA. 2014;312(23):2510–20.
Huynh T, et al. Aspirin, warfarin, or the combination for secondary prevention of coronary events in patients with acute coronary syndromes and prior coronary artery bypass surgery. Circulation. 2001;103(25):3069–74.
Taylor FC, Cohen H, Ebrahim S. Systematic review of long term anticoagulation or antiplatelet treatment in patients with non-rheumatic atrial fibrillation. BMJ. 2001;322(7282):321–6.
Segal JB, et al. Prevention of thromboembolism in atrial fibrillation: a meta-analysis of trials of anticoagulants and antiplatelet drugs. J Gen Intern Med. 2000;15(1):56–67.
Hart RG, et al. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131(7):492–501.
Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–67.
Dogliotti A, Paolasso E, Giugliano RP. Current and new oral antithrombotics in non-valvular atrial fibrillation: a network meta-analysis of 79 808 patients. Heart. 2014;100(5):396–405.
Cooper NJ, et al. Mixed comparison of stroke prevention treatments in individuals with nonrheumatic atrial fibrillation. Arch Intern Med. 2006;166(12):1269–75.
Andersen LV, et al. Warfarin for the prevention of systemic embolism in patients with non-valvular atrial fibrillation: a meta-analysis. Heart. 2008;94(12):1607–13.
Assiri A, et al. Mixed treatment comparison meta-analysis of aspirin, warfarin, and new anticoagulants for stroke prevention in patients with nonvalvular atrial fibrillation. Clin Ther. 2013;35(7):967–984. e2.
Lip GY, Edwards SJ. Stroke prevention with aspirin, warfarin and ximelagatran in patients with non-valvular atrial fibrillation: a systematic review and meta-analysis. Thromb Res. 2006;118(3):321–33.
Cameron C, et al. Systematic review and network meta-analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open. 2014;4(6):e004301.
Liu X, et al. Warfarin compared with aspirin for older Chinese patients with stable coronary heart diseases and atrial fibrillation complications. Int J Clin Pharmacol Ther. 2014;52(6):454–9.
Burton C, et al. The safety and adequacy of antithrombotic therapy for atrial fibrillation: a regional cohort study. Br J Gen Pract. 2006;56(530):697–702.
Aguilar MI, Hart R. Antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2005;19(4):CD001925.
Uchiyama S, et al. Aspirin for stroke prevention in elderly patients with vascular risk factors: Japanese primary prevention project. Stroke. 2016;47(6):1605–11.
Wiviott SD, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
Zhou YH, et al. Effects of combined aspirin and clopidogrel therapy on cardiovascular outcomes: a systematic review and meta-analysis. PLoS One. 2012;7(2):e31642.
Diener HC, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–7.
Gandhi S, et al. Comparison of dual-antiplatelet therapy to mono-antiplatelet therapy after Transcatheter aortic valve implantation: systematic review and meta-analysis. Can J Cardiol. 2015;31(6):775–84.
Leonardi-Bee J, et al. Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke. 2005;36(1):162–8.
Halkes PH, et al. Dipyridamole plus aspirin versus aspirin alone in secondary prevention after TIA or stroke: a meta-analysis by risk. J Neurol Neurosurg Psychiatry. 2008;79(11):1218–23.
Warkentin AE, et al. Bleeding risk in randomized controlled trials comparing warfarin and aspirin: a systematic review and meta-analysis. J Thromb Haemost. 2012;10(4):512–20.
Britton M, Helmers C, Samuelsson K. High-dose acetylsalicylic acid after cerebral infarction. A Swedish cooperative study. Stroke. 1987;18(2):325–34.
Sam C, et al. Warfarin and aspirin use and the predictors of major bleeding complications in atrial fibrillation (the Framingham heart study). Am J Cardiol. 2004;94(7):947–51.
Coleman CI, et al. Effect of pharmacological therapies for stroke prevention on major gastrointestinal bleeding in patients with atrial fibrillation. Int J Clin Pract. 2012;66(1):53–63.
Connolly BJ, et al. Aspirin therapy and risk of subdural hematoma: meta-analysis of randomized clinical trials. J Stroke Cerebrovasc Dis. 2013;22(4):444–8.
Stroke prevention in atrial fibrillation investigators. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet. 1994;343(8899):687–91.
Lin L, et al. Clinical and safety outcomes of oral Antithrombotics for stroke prevention in Atrial fibrillation: a systematic review and network meta-analysis. J Am Med Dir Assoc. 2015;16(12):1103.e1–1103.e19.
The American geriatrics society 2015 beers criteria update expert panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-46.
American geriatrics society 2012 beers criteria update expert panel. American geriatrics society updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–31.
O'Mahony D, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8.
Piepoli MF, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and Other Societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & rehabilitation (EACPR). Atherosclerosis. 2016;252:207–74.
ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials. 2013;36(2)555–64.
Slabaugh SL, et al. Prevalence and risk of polypharmacy among the elderly in an outpatient setting: a retrospective cohort study in the Emilia-Romagna region, Italy. Drugs Aging. 2010;27(12):1019–28.
Funding
The PRIMA-eDS study was supported by a grant from the European Commission within the 7th Framework Programme (Grant No. 305388–2). The work of YVM was also supported by a grant from the NIHR Greater Manchester Primary Care Patient Safety Translational Research Centre. The publication charge was funded by the University of Witten/Herdecke.
Availability of data and materials
The data supporting the conclusions of this article is included within the article (and its additional files).
About this supplement
This article has been published as part of BMC Geriatrics Volume 17 Supplement 1, 2017: The Evidence Base of Frequently prescribed drugs in older Patients: A series of systematic reviews as a basis for recommendations in the PRIMA-eDS-tool to reduce inappropriate polypharmacy. The full contents of the supplement are available online at https://bmcgeriatr.biomedcentral.com/articles/supplements/volume-17-supplement-1.
Author information
Authors and Affiliations
Contributions
ARG, AS, and YM conceptualised the SR and performed the database searches. AR and MM selected the studies. AR and MM conducted data extraction and quality appraisal supported by CS and MK. ARG, AS, IK and MM developed the recommendations. AR and MM drafted the manuscript. AE provided inputs to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Search string search 1 and 2. (DOCX 102 kb)
Additional file 2:
Overlap** studies. (DOCX 211 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Meinshausen, M., Rieckert, A., Renom-Guiteras, A. et al. Effectiveness and patient safety of platelet aggregation inhibitors in the prevention of cardiovascular disease and ischemic stroke in older adults – a systematic review. BMC Geriatr 17 (Suppl 1), 225 (2017). https://doi.org/10.1186/s12877-017-0572-7
Published:
DOI: https://doi.org/10.1186/s12877-017-0572-7