Abstract
Aim
This study aimed to compare the left ventricular (LV) systolic and diastolic parameters and left atrial (LA) mechanical functions of individuals engaging in recreational sports and resistance exercises on a weekly basis.
Methods
A total of 43 male amateur athletes were included in this study, of which 24 performed resistance exercises (REs) (29.70 ± 8.74 year, weight: 81.70 ± 12.64 kg, height: 176.05 ± 7.73 cm, BMI: 27.64 ± 4.97 kg/m2), and 19 participated in recreational football training and were included in the recreational sports group (31.73 ± 6.82 year, weight: 86.00 ± 18.52 kg, height: 178.62 ± 4.95 cm, BMI: 25.55 ± 3.42 kg/m2). The exercises were standardized according to the weekly exercise frequency and volume. After recording the participants’ demographic information, the LV systolic and diastolic parameters and LA mechanical functions were measured using echocardiography (ECHO) and Tissue Doppler Imaging.
Results
Significant differences were observed in various cardiac parameters between the recreational sports group (REG) and resistance exercise Group (RSG). Specifically, the left ventricular (LV) diastolic diameter, LV end diastolic volume index (LVEDVi), and stroke volume index were notably higher in the REG compared to the RSG (t = 2.804, p = .010, effect size (ES) = 2.10; t = 3.174, p = .003, ES = 0.98; t = 3.36, p = .002, ES = 1.02, respectively). Notably, the RSG exhibited higher values for LV mass index (LVMi) and isovolumic relaxation time (IVRT) than the REG (t = 2.843, p = .007, ES = 0.87; t = 2.517, p = .016, ES = 0.76) in terms of LV systolic and diastolic parameters. Regarding left atrial (LA) mechanics, the REG demonstrated increased LA total emptying volume index, LA maximum volume index, LA volume before systole measured at the onset of the p-wave index, and conduit volume index compared to RSG (t = 2.419, p = .020, ES = 0.75; t = 2.669, p = .011, ES = 0.81; t = 2.111, p = .041, ES = 0.64; t = 2.757, p = .009, ES = 0.84, respectively).
Conclusion
Our study revealed significant variations in LV and LA functions between REG and RSG. Our data suggest that REs led to substantial cardiac remodeling, altering myocardial structure and function. In contrast, the effect of recreational exercise on cardiac adaptation was less pronounced than that of resistance exercise. Consequently, we propose that individuals engaging in recreational exercise should consider modalities that impose higher cardiovascular demand for more effective cardiac conditioning.
Similar content being viewed by others
Introduction
Physical fitness and cardiovascular health are of paramount importance for athletes seeking to optimize their performance and overall well-being [1]. Cardiovascular fitness plays a critical role in an athlete’s ability to exert maximal effort. While structured training programs and rigorous workouts are essential components of an athlete’s routine, the role of recreational exercises in improving cardiovascular fitness has gained considerable attention. Recreational activities play a vital role in promoting physical fitness and overall well-being among individuals, including amateur athletes [2]. Recreational sports involve physical movements that occur during leisure time and trigger physiological bodily systems. These exercises include activities that are performed regularly, whether in adulthood or youth, and at the amateur or professional level [3]. As part of a larger spectrum of physical activity choices, individuals participate in recreational activities to promote their emotional and mental well-being by emphasizing enjoyment and relaxation, in contrast to exercises that focus on structured physical activity [3]. Engaging in regular recreational activities has been associated with numerous cardiovascular benefits, including enhanced exercise capacity and a reduced risk of cardiovascular diseases [4, 5]. Although the benefits of recreational exercise on cardiovascular fitness have been widely acknowledged [6,7,8], their specific effects on ventricular systolic and diastolic parameters and left atrial (LA) mechanical functions in amateur athletes have not been thoroughly investigated.
Over the last decade, growing evidence has shown that recreational football is a multifaceted exercise that offers a range of advantages beyond structured training sessions. Milanović et al. [9] meta-analyzed 17 studies and reported that recreational football produces large improvements in maximal oxygen consumption compared to strength training and no-exercise controls. Another meta-analysis demonstrated multiple broad-spectrum benefits of recreational football on health-related physical fitness compared with non-exercise controls, including improvements in blood pressure, resting heart rate, fat mass, low-density lipoprotein cholesterol, and countermovement jump performance [7]. Such activities provide an opportunity for athletes to engage in physical activities that are enjoyable and less regimented, thereby promoting mental well-being and reducing stress levels [1, 8]. Moreover, recreational football often involves dynamic movement patterns, varying intensities, and different energy systems, leading to a diverse set of physiological stimuli. This diversity in exercise patterns can contribute to improved central hemodynamics, as it challenges the cardiovascular system in different ways, thereby promoting adaptability and efficiency. However, the specific effects on the ventricular and atrial function parameters require further investigation.
It has been reported that recreational exercises may influence ventricular systolic and diastolic parameters, as well as LA mechanical functions [10, 11]. Physical activity has been found to result in an increase in LV end-diastolic volume (LVEDV). Additionally, it tends to augment the total peripheral vascular resistance (TPR) as well as blood flow towards the extremities [12]. Yet, little is known about the type of exercise performed. Traditional high-load (HL) resistance exercise (RE) routines, comprising 8–12 repetitions, at approximately 70–80% of one repetition maximum (1RM), have been shown to generate adaptations that lead to muscle improvement [13]. Both high-load (HL) and moderate-load (ML) exercise protocols comprising of 25–35 repetitions (approximately 30–50% of IRM) performed until volitional fatigue were found to stimulate hypertrophic adaptations [14]. Thus, regular exercise has been associated with numerous cardiovascular benefits, including improved cardiac function, increased cardiac output, and enhanced exercise capacity [15]. Although aerobic exercise is widely recognized for its cardiovascular benefits [15], there is growing interest in investigating the comparative effectiveness of engagement in regular recreational football and resistance exercises (REs) on cardiac adaptation. Recreational football combines the elements of both aerobic and anaerobic exercises, making it an appealing exercise modality to explore along with RE. Comparing the effects of RE and recreational football on cardiac adaptation allows for a comprehensive understanding of the potential cardiovascular adaptations associated with these exercise modalities. Therefore, this study aimed to investigate the potential effect of recreational football training compared to resistance exercises on cardiac adaptation among amateur athletes. Specifically, we examined the effect of recreational exercises on ventricular systolic and diastolic parameters, as well as LA mechanical function using echocardiography (ECHO) and Tissue Doppler Imaging (TDI). The ECHO and TDI are valuable tools for assessing cardiac adaptations because they allow the evaluation of structural and functional changes in the heart [16]. It is a well-known method for imaging changes in shape, thickness, ventricular and aortic diameters, velocity, and many diagnostic factors in clinical diseases [16]. Understanding the effects of exercise on ECHO parameters is important for optimizing exercise prescriptions, monitoring cardiovascular health, and guiding clinical decision making. Additionally, understanding these distinct effects can provide valuable insights for designing exercise programs tailored to the specific goals and needs of amateur athletes. Moreover, it can contribute to the existing body of knowledge on the benefits of recreational exercise and resistance training on cardiac function, potentially informing training recommendations for individuals seeking to improve their cardiovascular fitness and overall well-being.
Methods
Study design and participants
In this cross-sectional study, the required sample size was estimated based on a previous study conducted by Kurtoğlu et al. [17] in which the ECHO findings of football players was reported to be 50.91 ± 4.01 mm. Thus, we performed a power analysis using a significance level (α) of 0.05, power (1-β) of 0.80, and effect size of 0.86. The analysis indicated that a minimum of 18 participants per group would be needed in our study.
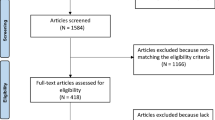
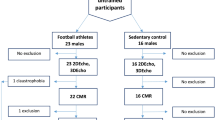
A nonprobability convenience sampling method was used in this study. The inclusion criteria were male individuals without pre-existing health conditions who engaged in resistance training and recreational football at least twice weekly for a duration of one year. Participants who engaged in resistance training that involved the progressive use of various resistive loads, movement velocities, and training modalities were included in the resistance exercise group (REG). The training modalities include weight machines, free weights (e.g., barbells and dumbbells), elastic bands, medicine balls, or plyometrics performed twice a week. Participants who engaged in recreational football training for at least twice a week were included in the recreational sports group (RSG). We excluded male participants with hypertension, tachycardia or bradycardia, developmental disorders (e.g., thyroid and heart valve disorders), and those who had already undergone surgery for a heart problem. We also excluded participants who reported any history of sleep disorders, who did not comply with the researcher’s instructions, or who were found to have health problems during testing. In this context, a total of 24 male participants who performed REs (REG) for at least 2 days a week, and 19 male participants who played football for recreational purposes (RSG) at least 2 days a week were included in our research. The study participants self-reported an average duration of sports experience of 4 ± 2.5 years. All participants were assessed after 8 h of sleep and were instructed not to eat or drink other than water for at least 3 h before taking part in the testing. The flow diagram of the study is illustrated in Fig. 1.
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki and was approved by both Bandirma Onyedi Eylul University (BU) and the Human Research Ethics Committee of BU (approval number: 2022/222). Written informed consent was obtained after participants were informed of the purpose and details of the study.
Study procedure and measurement
Body surface area
After determining the demographic characteristics of the participants, their height and weight were measured and used to calculate body mass index (BMI) using the following formula: body weight (kg)/height squared (m²). Body surface area (BSA) was calculated according to the following formula: body weight (kg) 0.425 × height (cm) 0.725 × 0.007184 [18].
Blood pressure
Blood pressure was measured by a cardiologist after 9 min of complete passive rest following the standardized method of measurement [19]. Systolic and diastolic blood pressures were measured using a stethoscope and a sphygmomanometer (Erka Perfect Aneroid / Germany).
Echocardiographic measurements (ECHO)
The ECHO evaluations were performed by a specialist cardiologist in the cardiology clinic. All tests were performed at the same time of the day (morning). Participants were asked to take eight hours of sleep before the measurements. All ECHO examinations were performed using the Vivid T8 device and 3ScRS transducer (GE Medical System, Milwaukee). All measurements were performed according to the recommendations of the American Society of Echocardiography Guidelines [20]. Echocardiographic images and recordings were obtained in the parasternal long-axis, apical four-chamber, and apical two-chamber views in the left decubitus position and at rest [21]. The following 2-dimensional and M-mode echocardiographic parameters were measured: aortic diameters in systole (ADs) and diastole (ADd), left ventricular end-diastolic diameter (LVDd in mm), left ventricular end-systolic diameter (LVDs), interventricular septal thickness (IVST), and posterior wall thickness (PWT).
The left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), stroke volume (SV), and ejection fraction (EF) were measured in the apical four-chamber view by the modified Simpson method [22]. Pulsed wave (PW), early diastolic flow velocity (E), late diastolic flow parameter (A), E/A ratio, ejection time (ET), isovolumic relaxation time (IVRT), isovolumic contraction time (IVCT), and transmissible flow parameters were measured. Tissue Doppler imaging of annulus motion was measured from lateral mitral annulus and peak early systolic (Sm), peak early diastolic (Em), and peak late diastolic (Am) velocities.
Tissue doppler imaging (TDI)
The LV lateral wall in apical 4-chamber views from the mitral lateral annulus was used for TDI. A clear image signal was obtained by adjusting the TDI filter and Nyquist cutoff to a value of 16–20 cm/s. The left ventricular mass index (LVM-I) was calculated using the Devereux formula [23]. The aortic systolic and diastolic diameters were obtained from the recordings made after the insertion of the M-mode rod through the area of the ascending aorta 3 cm distal to the aortic valve. The systolic and diastolic diameters were measured in the area corresponding to the R peak of the ECG from the point of maximum forward motion in the aortic curve. The measurements were repeated for three heartbeats and the average value was determined [24]. The aortic strain (AS) and aortic distensibility (AD) were used as aortic elasticity parameters. The following formula was used to calculate these parameters:
Aortic Strain (%) = [(systolic diameter-diastolic diameter) x 100] /diastolic diameter.
Distensibility (10 − 6.cm-2.dyn-1) = 2 (aortic strain ) / (systolic pressure-diastolic pressure) [25].
Left atrial volumes were calculated from apical four-chamber and two-chamber views using the biplane field length method. The maximum left atrial volume (LAVmax) was measured when the mitral valve was fully open, minimum left atrial volume (LAVmin) was measured when the mitral valve was fully closed and left atrial volume before systole was measured at the onset of the p wave (LAVp) on the electrocardiogram. All measurements were repeated during three consecutive heartbeats and averaged. The LAVmax index was determined by dividing the maximum volume of the left atrium (LAVmax) by body surface area. All volumes were corrected by dividing by the LAVmax index. Left atrial function was determined using the following formula [26].
-
LA passive emptying volume (LAPEV) = LAV max – LAVp.
-
LA passive emptying fraction (LAPEF) = LAPEV / LAVmax.
-
LA active emptying volume (LAAEV) = LAVp – LAVmin.
-
LA active emptying fraction (LAAEF) = LAAEV / LAVp.
-
LA total emptying volume (LATEV) = LAVmax - LAVmin.
-
LA total emptying fraction (LATEF) = LATEV / LAVmax.
-
Conduction volume (CV) = Left ventricular stroke volume - (LAVmax-LAVmin).
All ECHO measurements were performed by two independent experts. The similarity between the data in both analyzes was determined as 96.8%.
Statistical analysis
Statistical analyses were performed using SPSS 25.0 (SPSS, Inc., Chicago, IL, USA). Normality analysis of the data was performed using the Shapiro-Wilk test. Levene’s test was performed to determine the homogeneity of variances. The data were found to be normally distributed. For comparison of the mean differences between the groups in the outcomes of interest, the independent sample t-test was used. GraphPad Prism 8 was used to visualize the data. Cohen’s d effect size (ES) with 95% confidence interval was calculated to define the magnitude of pairwise comparisons for pre- and post-test. The ES magnitude was defined as follows: <0.2 = trivial, 0.2 to 0.6 = small effect, > 0.6 to 1.2 = moderate effect, > 1.2 to 2.0 = large effect, and > 2.0 = very large [27]. Statistical significance was set at an alpha level of 0.05.
Results
Table 1 shows the demographic characteristics, sports experiences and weekly exercise duration of the participants. The study findings revealed no significant variance among the groups in age, weight, height, sports experience, training frequency, BSA, BMI, Systolic blood pressure (SBP), and diastolic blood pressure (DBP) outcomes (p > .05). These results suggest that the groups share similar characteristics.
Table 2 shows the results of the routine ECHO parameters of the participants. It was observed that the values of the LV diastolic diameter (LVDd) (t = 2.804, p = .010, ES = 2.10), LVEDVi (t = 3.174, p = .003, ES = 0.98), and stroke volume index (SVi)(t = 3.36, p = .002, ES = 1.02) and LV mass index (LVMi) (t = 2.843, p = .007, ES = 0.87) were significantly higher in the REG than in the RSG (Fig. 2). However, there were no significant difference between the groups in ADd, ADs, LVDs, LVESVi, IVST, and PWT (p > .05). Figure 2. Description of the ECHO parameters across the groups.
LV systolic and diastolic parameters of the study participants are compared in Table 3. It was found that isovolumic relaxation time (IVRT) (t = 2.517, p = .016, ES = 0.76) was higher in the REG. However, no statistical significant differences were observed in the early and late diastolic flow parameter (E, A), ejection time (ET), isovolumic contraction time (IVCT), peak early diastolic (E), peak late diastolic (Am), peak early systolic (Sm), left ventricular ejection fraction (LVEF), E/A Ratio, and E/Em ratio (p > .05).
The LA functions of the participants reported in Table 4. Figure 3 shows a higher mean value of the LA total emptying volume index (LATEVi), LA maximum volume index (LAmaxi), LA volume before systole measured at the onset of the p wave index (LAPi), and conduit volume index (CVi) in the REG than in the RSG (p < .05). However, there was no significant difference in left atrium passive emptying volume index (LAPEVi), LAPEFi, LAAEVi, LAAEFi, LATEFi.
Table 5 displays the results of aortic strain and distensibility among the participants. The statistical analysis reveals that there was no significant difference observed between the aortic strain and distensibility results among the participants (p > .05).
Discussion
In our investigation, we compared the LV systolic and diastolic parameters, as well as the left atrial (LA) mechanical functions, between individuals engaged in REs and those participating in recreational sports, with both groups maintaining similar weekly exercise durations. Primary outcomes illustrated that the resistance exercise group (REG) demonstrated elevated left ventricular end-diastolic diameter (LVDd), left ventricular end-diastolic volume index (LVEDVi), left ventricular mass index (LVMi), stroke volume index (SVi), and isovolumic relaxation time (IVRT) when compared to the recreational sports group (RSG). Furthermore, in regard to LA mechanical functions, late diastolic mitral annular velocity (LATEvi), maximum left atrial volume index (LAmaxi), left atrial pressure index (LAPi), and conduit volume index (CVi) were significantly augmented in the REG [33, 34]. To our knowledge, this study represents the first endeavor to elucidate the distinctions between left ventricular (LV) systolic and diastolic parameters and LA mechanical functions in individuals participating in both RE and recreational soccer.
Long-term and consistent exercise has been established to promote physiological adaptations within the cardiovascular system [28], [29], enabling acute responsiveness to increased demands imposed by skeletal muscles during physical activity [30]. As exercise that challenges the cardiovascular system becomes a habitual practice, adaptive alterations, such as cardiac hypertrophy and augmented contractility, are stimulated [30]. Current guidelines advocate for moderate exercise engagement, totaling a minimum of 150 min per week [31]. Nonetheless, the specific exercise modality that provides optimal health benefits to sedentary individuals remains a topic of ongoing debate. Our data highlight that REG participants exhibited superior LVDd, LVEDV, and SVi compared with their RSG counterparts, potentially as a consequence of elevated blood pressure, facilitating augmented skeletal muscle blood flow [32], diminished peripheral resistance, and enhanced cardiac contractile capacity [33]. Chronic exercise engagement prompts hemodynamic adaptations, normalizing LV wall tension via internal size adjustments and LV hypertrophy (LVH), while preserving or even elevating LV systolic and diastolic function [31] [34]. A previous study highlighted an increase in the LVEDd during heavy RE. It has been found that heavy RE significantly increases arterial blood pressure [35]. Keul et al. found that LVDd was higher in strength athletes than in long- and middle-distance runners [36]. Furthermore, the potential mechanism to increase end-diastolic volume is enhanced venous return to the heart and decreased resting heart rate, resulting in prolongation of diastole and increased diastolic filling. As a result, there is an increase in cardiac stroke volume [37]. Furthermore, it is known that long-term exercise increases the number of blood vessels in the muscles, resulting in a decrease in peripheral resistance and an increase in contractility [38].
It has been found that the LVMi was statistically elevated in the REG, which, considering equivalent LV wall thickness between the groups, is attributed to excessive LVEDV in the REG [39]. Corroborating the Morganroth hypothesis [40], our findings suggest that endurance training elicits eccentric LVH owing to volume loading and increased diastolic wall stress, whereas strength training predominantly induces concentric LVH owing to compressive loading and augmented systolic wall stress. Furthermore, our study revealed significant LA remodeling in the REG, potentially ascribed to elevated LVMi and LVEDV [41]. Although the mechanisms underpinning atrial adaptation to exercise remain incompletely understood [42], emerging evidence suggests that LA may be more responsive to exercise than LV [43]. The adaptive capacity of LA serves to modulate LV filling pressures, both at rest and during physical exertion [44].
Notably, our analysis revealed no significant differences between the groups regarding aortic stiffness. Established cardiovascular risk factors, including age, diabetes, hypertension, smoking, and dyslipidemia, exacerbate arterial stiffness and promote atherosclerosis [45]. Nevertheless, regular engagement in either aerobic or resistance exercise appears to exert comparable salutary effects on cardiovascular stiffness and elasticity [46, 47].
Despite the absence of significant differences in diastolic echocardiographic parameters between the groups, IVRT was within normal limits but protracted in the REG, possibly due to the heightened LVMi [48]. This may also explain the observed elongation of IVRT in the absence of pathological diastolic dysfunction [48]. In addition, the higher LATEVi, LAmaxi, and LAPi parameters suggest that RE may have a significant effect on LA. However, during RE, blood pressure and pulse fluctuations may occur because of vagal activity and increased intrathoracic pressure. Although no disturbance was noted in the diastolic parameters, further investigations should be conducted to determine whether this is a promoting factor, particularly for arrhythmias of atrial origin.
In summary, our findings emphasize the distinctive cardiac adaptations associated with different exercise modalities, with resistance training in particular inducing notable alterations in LV and LA parameters [49, 50]. While these adaptations are generally physiological and beneficial, ongoing surveillance is prudent, particularly to discern any potential pro-arrhythmic effects, especially of atrial origin, under certain conditions [51]. Further research is warranted to substantiate these observations and elucidate the long-term clinical implications of exercise-induced cardiac adaptations.
This study has certain limitations that should be considered when interpreting the findings. First, our investigation focused on male individuals who engaged in recreational sports and those who performed RE. Therefore, the generalizability of the results to other populations or individuals participating in different types of exercises may be limited. Future studies should explore the impact of various exercise modalities on cardiac parameters to obtain a more comprehensive understanding. Second, it is important to acknowledge that our study design was cross-sectional, which does not explain the causation underlying adaptation. Longitudinal cohort studies with extended follow-up periods are necessary to assess the long-term effects of exercise on the cardiac structure and function. Such studies would allow the investigation of potential structural changes in the heart over time and provide valuable insights into the progression of cardiovascular adaptations associated with exercise. Third, this study focused on the effects of specific training but did not consider individual differences in training intensity, duration, frequency, and baseline level of cardiorespiratory fitness among the participants. This variability in training could potentially have influenced the outcomes of this study. These limitations highlight the need for further research that includes a more comprehensive evaluation of training variables and baseline fitness levels, which can provide a greater understanding of the mechanisms of cardiac modulation and other relevant outcomes. Despite these limitations, our study contributes to the existing body of knowledge regarding the effectiveness of RE and recreational football training in cardiac adaptation.
Conclusion
According to the findings of our study, we observed notable variations in the LV and LA functions in the REG compared to the RSG. These disparities were evident despite the weekly exercise frequency and volume being comparable between the cohorts. Our data suggest that resistance exercises (REs) induce significant cardiac remodeling, which is characterized by alterations in myocardial structure and function. While recreational exercises are documented to enhance overall physical fitness levels [7], our findings indicate that their impact on cardiac adaptation is not as pronounced as that observed with resistance exercises. Consequently, we propose that individuals engaging in exercise for recreational purposes should consider incorporating exercise modalities that impose greater cardiovascular demand, thereby promoting more substantial cardiac conditioning. Furthermore, we advocate the involvement of an exercise physiologist or a clinical exercise specialist in the design and supervision of exercise regimens for these individuals to ensure safety and maximal therapeutic benefit.
Data Availability
The data is not publicly available because further research is being done and more manuscripts are being prepared. Data for the current study will be available upon reasonable request from the principal investigator or corresponding author.
Abbreviations
- A:
-
Late diastolic flow parameter
- Am:
-
Peak late diastolic
- AD:
-
Aortic distensibility
- ADd:
-
Aortic diameter diastole
- ADs:
-
Aortic diameter systole
- AS:
-
Aortic strain
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- CVi:
-
Conduit volume index
- DBP:
-
Diastolic blood pressure
- E:
-
Early diastolic flow velocity
- Em:
-
Peak early diastolic
- ECHO:
-
Echocardiography
- ET:
-
Ejection time
- IVCT:
-
Isovolumic contraction time
- IVRT:
-
Isovolumic relaxation time
- IVST:
-
Interventricular septal thickness
- LVEF:
-
Left ventricular ejection fraction
- LVMi:
-
Left ventricular mass index
- LAAEF:
-
Left atrium active emptying fraction index
- LAAEV:
-
Left atrium active emptying volume index
- LAPEF:
-
Left atrium passive emptying fraction index
- LAPEV-I:
-
Left atrium passive emptying volume index
- LATEF-I:
-
Left atrium total emptying fraction index
- LATEFSV-I:
-
Left atrium total emptying fraction index
- LVDd:
-
Left ventricular end-diastolic diameter
- LVDs:
-
Left ventricular end-systolic diameter
- LVEDVi:
-
Left ventricular end-diastolic volume index
- LVESVi:
-
Left ventricular end-systolic volume index
- PWT:
-
Posterior wall thickness
- REG:
-
Resistance exercise group
- RSG:
-
Recreational sports group
- Sm:
-
Peak early systolic
- SVi:
-
Stroke volume index
- SBP:
-
Systolic blood pressure
- TF:
-
Training frequency
References
DeVries HA. Physiology of exercise for physical education and athletics. 1974.
Macovei S, Tufan AA, Vulpe BI. Theoretical approaches to building a healthy lifestyle through the practice of physical activities. Procedia-Social and Behavioral Sciences. 2014;117:86–91.
Caglar E, Canlan Y, Demir M. Recreational Exercise motives of adolescents and young adults. J Hum Kinetics. 2009;22:83–9.
Slattery ML, Jacobs DR, Nichaman MZ. Leisure time physical activity and coronary heart disease death. The US Railroad Study. Circulation. 1989;79:304–11.
Iwase H, Tanaka-Mizuno S, Takashima N, Kadota A, Matsui K, Nakamaura Y, et al. Relationship of leisure-time and household physical activity level and type with cardiovascular disease: secondary analysis of the Takashima Study data. BMC Cardiovasc Disord. 2022;22:132.
Blair SN, Morris JN. Healthy hearts—and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253–6.
Milanović Z, Pantelić S, Čović N, Sporiš G, Mohr M, Krustrup P. Broad-spectrum physical fitness benefits of recreational football: a systematic review and meta-analysis. Br J Sports Med. 2019;53:926–39.
Szabo A, Ábrahám J. The psychological benefits of recreational running: a field study. Psychol Health Med. 2013;18:251–61.
Milanović Z, Pantelić S, Čović N, Sporiš G, Krustrup P. Is recreational Soccer Effective for improving V O 2 max? A systematic review and Meta-analysis. Sports Med. 2015;45:1339–53.
Martinez MW, Kim JH, Shah AB, Phelan D, Emery MS, Wasfy MM, et al. Exercise-induced cardiovascular adaptations and approach to exercise and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:1453–70.
Breisch EA, White FC, Nimmo LE, McKirnan MD, Bloor CM. Exercise-induced cardiac hypertrophy: a correlation of blood flow and microvasculature. J Appl Physiol. 1986;60:1259–67.
Hassanpour Dehkordi A, Khaledi Far A. Effect of Exercise Training on the quality of Life and Echocardiography parameter of systolic function in patients with chronic heart failure: a Randomized Trial. Asian J Sports Med. 2015;6.
Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW. Strength and hypertrophy adaptations between Low- vs. high-load resistance training: a systematic review and Meta-analysis. J Strength Conditioning Res. 2017;31:3508–23.
Lasevicius T, Schoenfeld BJ, Silva-Batista C, Barros T, de Aihara S, Brendon AY. Muscle failure promotes Greater muscle hypertrophy in low-load but not in high-load resistance training. J Strength Conditioning Res. 2022;36:346–51.
Kiflom S, Enyew D, Ayalew A, Hailu A, Gebretensay M, Gebrehawerya G. Effect of aerobic exercise on physiological and left ventricular echocardiographic characteristics of non-athletic adult males in northern Ethiopia. J Sports Med Phys Fit. 2022;62.
Wong V, Spitz RW, Bell ZW, Viana RB, Chatakondi RN, Abe T, et al. Exercise induced changes in echo intensity within the muscle: a brief review. J Ultrasound. 2020;23:457–72.
Kurtoğlu A, Kurtoğlu E, Konar N, Çar B, Eken Ö, Prieto-González P, et al. Comparison of echocardiographic parameters of amputee football players with active football players and sedentary individuals. BMC Sports Science Medicine and Rehabilitation. 2023;15:41.
Zafrir B, Salman N, Crespo-Leiro MG, Anker SD, Coats AJ, Ferrari R, et al. Body surface area as a prognostic marker in chronic heart failure patients: results from the Heart failure Registry of the heart failure association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:859–68.
Johnson KA, Partsch DJ, Gleason P, Makay K. Comparison of two home blood pressure monitors with a Mercury Sphygmomanometer in an Ambulatory Population. Pharmacotherapy. 1999;19:333–9.
Lang R, Bıerıg M, Devereux R, Flachskampf F, Foster E, Pellıkka P, et al. Recommendations for chamber quantification☆. Eur J Echocardiography. 2006;7:79–108.
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic Assessment of the right heart in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713.
U**o K, Barnes ME, Cha SS, Langins AP, Bailey KR, Seward JB, et al. Two-dimensional echocardiographic methods for Assessment of Left Atrial volume. Am J Cardiol. 2006;98:1185–8.
Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–8.
Stefanadıs C, Stratos C, Boudoulas H, Kourouklıs C, Toutouzas P. Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J. 1990;11:990–6.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension. 2001;37:1236–41.
Yilmaz M, Arican Ozluk FO, Akgumus A, Peker T, Karaagac K, Vatansever F, et al. Left atrial mechanical functions in patients with the metabolic syndrome. Acta Cardiol. 2013;68:133–7.
Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in Sports Medicine and Exercise Science. Med Sci Sports Exerc. 2009;41:3–12.
Moghetti P, Bacchi E, Brangani C, Donà S, Negri C. Metabolic Effects of Exercise. 2016. p. 44–57.
Lavie CJ, Arena R, Swift DL, Johannsen NM, Sui X, Lee DC, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circul Res. 2015;117:207–19.
Uzun M. Cardiovascular System and Exercise. J Cardiovasc Nurs. 2016;7:48–53.
Galderisi M, Cardim N, D’Andrea A, Bruder O, Cosyns B, Davin L, et al. The multi-modality cardiac imaging approach to the Athlete’s heart: an expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J - Cardiovasc Imaging. 2015;16:353–353r.
Mang ZA, Ducharme JB, Mermier C, Kravitz L, de Castro Magalhaes F, Amorim F. Aerobic adaptations to Resistance Training: the role of Time under Tension. Int J Sports Med. 2022;43:829–39.
Rivera-Brown AM, Frontera WR. Principles of Exercise Physiology: responses to Acute Exercise and Long-term Adaptations to Training. PM&R. 2012;4:797–804.
Menapace FJ, Hammer WJ, Rıtzer TF, Kessler KM, Warner HF, Spann JF, et al. Left ventricular size in competitive weight lifters. Med Sci Sports Exerc. 1982;14:72–5.
Park W, Jung W-S, Hong K, Kim Y-Y, Kim S-W, Park H-Y. Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 weeks on body composition, cardiometabolic risk factors, blood pressure, arterial stiffness, and physical functions, among obese older men: a pilot study. Int J Environ Res Public Health. 2020;17:7233.
Keul J, Dickhuth HH, Simon G, Lehmann M. Effect of static and dynamic exercise on heart volume, contractility, and left ventricular dimensions. Circul Res. 1981;48:62–70.
Green HJ, Jones LL, Painter DC. Effects of short-term training on cardiac function during prolonged exercise. Med Sci Sports Exerc. 1990;22:488–93.
Prisby RD. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J Endocrinol. 2017;235:R77–100.
Halliday BP, Senior R, Pennell DJ. Assessing left ventricular systolic function: from ejection fraction to strain analysis. Eur Heart J. 2021;42:789–97.
Morganroth J. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82:521–4.
D’Andrea A, Riegler L, Golia E, Cocchia R, Scarafile R, Salerno G, et al. Range of right heart measurements in top-level athletes: the training impact. Int J Cardiol. 2013;164:48–57.
Iskandar A, Mujtaba MT, Thompson PD. Left Atrium size in Elite athletes. JACC: Cardiovasc Imaging. 2015;8:753–62.
Liu M, Sun M, Li L, Li P, Hou S, Li Z, et al. Left atrial function in young strength athletes: four-dimensional automatic quantitation study. Int J Cardiovasc Imaging. 2022;38:1929–37.
Maffeis C, Morris DA, Belyavskiy E, Kropf M, Radhakrishnan AK, Zach V, et al. Left atrial function and maximal exercise capacity in heart failure with preserved and mid-range ejection fraction. ESC Heart Failure. 2021;8:116–28.
Karakuş M, Akkurt S. Exercise and arterial stiffness. Turkish J Sports Med. 2017;52:025–35.
Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of Aerobic Exercise Training on the stiffness of central and peripheral arteries in Middle-Aged sedentary men. Jpn J Physiol. 2005;55:235–9.
Gómez-Cabello A, Ara I, González-Agüero A, Casajús JA, Vicente-Rodríguez G. Effects of Training on Bone Mass in older adults. Sports Med. 2012;42:301–25.
Smith VE, White WB, Karimeddini MK. Echocardiographic assessment of left ventricular diastolic performance in hypertensive subjects. Correlation with changes in left ventricular mass. Hypertension. 1987;9:80–4.
Pelliccia A. Physiologic left ventricular cavity dilatation in Elite athletes. Ann Intern Med. 1999;130:23.
Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, Parker RA, et al. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction. J Am Coll Cardiol. 2000;35:477–84.
Rowland T, Heffernan K, Jae SY, Echols G, Fernhall B. Tissue Doppler Assessment of ventricular function during Cycling in 7- to 12-yr-old boys. Med Sci Sports Exerc. 2006;38:1216–22.
Dergaa I, Chamari K, Zmijewski P, Saad HB. From human writing to artificial intelligence generated text: examining the prospects and potential threats of ChatGPT in academic writing. Biology of Sport. 2023;40:615–22.
Acknowledgements
We would like to thank Princess Nourah bint Abdulrahman University for supporting this project through Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R 286), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R 286), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All the authors contributed substantially to the manuscript. A.A, A.K, E.A, A.B, B.C, O.E and M.I.A contributed to the conception and analysis of the study. A.A, O.E conducted that statistical analysis of the data. A.A, A.K, E.A, A.B, B.C, O.E, and M.I.A were involved in preparation of the manuscript and reviewed the manuscript for important intellectual content. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the principles outlined in the Declaration of Helsinki and was approved by both Bandirma University (BU) and the Human Research Ethics Committee of BU (approval number: 2022/222). Informed consent was obtained from all study participants prior to their participation.
Consent for publication
Not applicable.
Conflict of interest
We declare that we have no financial or personal relationships among the authors that could potentially influence or bias the content of this study. None of the authors have any financial interests or conflicts of interest concerning the ChatGPT or NLM technologies discussed in this manuscript. Moreover, there have been no collaborations or consultations with individuals or organizations with financial or other interests in ChatGPT or NLM technologies [52].
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Akgümüş, A., Kurtoğlu, A., Aydın, E. et al. The insufficiency of recreational exercises in improving cardiovascular fitness: an investigation of ventricular systolic and diastolic parameters and left atrial mechanical functions. BMC Cardiovasc Disord 23, 486 (2023). https://doi.org/10.1186/s12872-023-03508-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03508-0