Abstract
Background
Zostera marina L., or eelgrass, is the most widespread seagrass species throughout the temperate northern hemisphere. Unlike the dry seeds of terrestrial plants, eelgrass seeds must survive in water, and salinity is the key factor influencing eelgrass seed germination. In the present study, transcriptome and proteome analysis were combined to investigate the mechanisms via which eelgrass seed germination was stimulated by low salinity, in addition to the dynamics of key metabolic pathways under germination.
Results
According to the results, low salinity stimulated the activation of Ca2+ signaling and phosphatidylinositol signaling, and further initiated various germination-related physiological processes through the MAPK transduction cascade. Starch, lipids, and storage proteins were mobilized actively to provide the energy and material basis for germination; abscisic acid synthesis and signal transduction were inhibited whereas gibberellin synthesis and signal transduction were activated, weakening seed dormancy and preparing for germination; cell wall weakening and remodeling processes were activated to provide protection for cotyledon protrusion; in addition, multiple antioxidant systems were activated to alleviate oxidative stress generated during the germination process; ERF transcription factor has the highest number in both stages suggested an active role in eelgrass seed germination.
Conclusion
In summary, for the first time, the present study investigated the mechanisms by which eelgrass seed germination was stimulated by low salinity and analyzed the transcriptomic and proteomic features during eelgrass seed germination comprehensively. The results of the present study enhanced our understanding of seagrass seed germination, especially the molecular ecology of seagrass seeds.
Similar content being viewed by others
Background
Seagrasses are marine angiosperms that form large shallow meadows with high ecological value [1,2,3,4,5]. However, seagrass species diversity and coverage have declined dramatically globally due to both natural and anthropogenic disturbance, which have caused the decline of the critical ecosystem globally [6, 7]. To halt and reverse such losses, attempts are already underway to restore seagrass meadows, various strategies have been adopted [6, 8,9,10,11,12], and seeding has become a widely used restoration method [9, 13, 14].
Zostera marina L., or eelgrass, is the most widespread seagrass species throughout the temperate northern hemisphere of the Pacific and Atlantic [15, 16], and is also one of the most threatened seagrass species. In recent years, much efforts of restoration based on eelgrass seeds have been carried out worldwide [6, 13, 14]. Current studies on eelgrass seed germination have focused on seed ecology, mainly on the effects of temperature, salinity, light, sediment type, burial depth, oxygen potential, and other factors, on seed germination [17]. Unlike the dry seeds of terrestrial plants, eelgrass seeds must survive in water [18, 19], and salinity is the key factor influencing eelgrass seed germination, low salinity promoted seed germination, while high salinity inhibited it [19, 20]. Nowadays, excessive rainfall caused by climatic anomalies may decrease the seawater salinity in seagrass beds in coastal zone [21, 22], which will affect seagrass seed germination. Seed germination is regulated both by the external environment and internal molecular mechanisms. Nonetheless, few studies have explored the molecular dynamics behind seagrass seed germination.
Seed germination is the beginning of the second cycle of a plant’s life [23]. Germination is the physiological process by which a seed begins to absorb water until the radicle emerges [24]. During terrestrial monocotyledon seed germination, the cotyledon and radicle are covered by a coleoptile and coleorhiza, respectively, and the coleorhiza and radicle grow out of the seed in sequence, following which the coleoptile is pushed upward to the surface [46]. It has been demonstrated that glycolysis was the main source of energy produced by respiration during early germination in some species [47,48,49]. According to our analysis results, eelgrass seed germination was no exception. Glycolysis was the enriched significantly pathway of persistently upregulated genes in the temporal expression analysis, which indicated that the glycolytic process was activated continuously during eelgrass seed germination and was the main energy source.
Lipids in seeds of higher plants can be used as energy sources during embryonic development [50]. During seed germination, mobilization of stored lipids begins with the breakdown of accumulated triacylglycerols in the oil bodies to free fatty acids and glycerol [51]. Acetyl Co-A produced by fatty acid β oxidation generates succinate in acetaldehyde vesicles via the glyoxylate cycle. Subsequently, succinate is exported to the mitochondria to participate in the TCA cycle, providing the energy and carbon skeleton for subsequent germination and seedling establishment [52]. KEGG pathway enrichment analysis in the present study identified several lipid metabolism-related pathways as significantly upregulated in both stages. In addition, temporal expression analysis identified one lipid metabolism pathway that was consistently upregulated; the results suggested that the breakdown of stored lipids was also a critical step in the germination process of eelgrass seeds. Seed storage proteins are the main source of amino acids in the early stages of seed germination, which can be used for the synthesis of subsequent enzymes and structural proteins, and are also vital for energy production [53,54,55,56]. Proteins in soybean seeds are degraded by protease and 26 S proteasome system, whereas rice seeds are degraded by protease during germination [49, 56]. Our combined transcriptomic and proteomic analysis results indicated that the degradation dynamics of storage proteins involved in eelgrass seed germination were similar to those of soybean seeds, implying that the 26 S proteasome system played a major role in their degradation. In addition, seed germination activates various physiological processes, which involve the resynthesis of a large number of enzymes, which could explain the significant activation of protein translation and processing processes.
GA and ABA-related gene and protein dynamics
Seed dormancy and germination cannot be regulated without phytohormones. ABA and GA have been demonstrated to be the two most critical factors [57]. ABA is associated with the induction and maintenance of dormancy, and GA is associated with seed germination promotion [57]. We analyzed the synthesis, degradation, and signal transduction of ABA and GA. Overall, we observed that some genes involved in ABA synthesis and positive signal transduction were repressed, some genes involved in ABA negative signal transduction were activated; and some genes involved in GA synthesis and positive signal transduction were activated.
Specifically, genes such as ZEP, CCD, ABF, and ABI5 were down-regulated significantly during the pre-germination stage, and PP2C genes were up-regulated significantly in both the pre-germination and germination stages. Based on genetic and functional studies in Arabidopsis, key components of the ABA biosynthetic pathway include ZEP/ABA1, which catalyzes the conversion of zeaxanthin to all-trans zeaxanthin, and NCED, belonging to a subfamily of CCD, which cleaves 9-cis xanthophylls to xanthoxin, a precursor of ABA [58, 59]. ABI5 is a bZIP TF and a core TF in ABA signaling that inhibits seed germination and maintains seed dormancy [60, 61]. ABRE binding factor (ABF) TFs regulate the expression of ABRE-dependent genes; therefore, ABF is also a positive regulator of ABA signaling, promoting seed dormancy and inhibiting seed germination [62]. PP2C is one of the core components of ABA signaling; it interacts with SnRK2s, leaving ABA signaling pathway in the “off” state [63, 64]. AOS is an important enzyme in the synthesis of jasmonic acid, which promotes seed germination by inhibiting ABA synthesis and promoting ABA inactivation [65, 68]. GA3ox catalyzes the final step of the GA biosynthesis pathway to produce active GA molecules [69]. GASA is a class of gene family induced by GA that encodes a small molecular polypeptide [70]. Rubinovich and Weiss [70]. observed that overexpression of the GA-inducible GASA4 gene in Arabidopsis promoted the response to GA, thereby facilitating flowering and seed germination.
Cell wall loosening and remodeling
Endosperm weakening is a crucial stage of the seed germination process. It facilitates radicle breaking through the endosperm and completion of the germination process [71]. Cell Wall Loosening factors (CWLFs) weaken the cell wall by encoding enzymes and non-enzymatic factors, resulting in weakening of the mechanical strength of the cell wall. Enzymatic CWLFs mainly include enzymes encoding the degradation of cellulose, hemicellulose and pectin [72,73,74,75]. Nonenzymatic CWLFs mainly include genes encoding expansin and genes related to ROS metabolism [75, 76]. According to our results, a large number of CWLF genes were significantly upregulated in two stages, such as genes encoding cellulose degradation (endoglucanase, beta-glucosidase); genes encoding hemicellulose degradation (beta-mannosidase; beta-galactosidase; beta-xylosidase; XTHs); genes encoding pectin degradation (pectinesterase; polygalacturonase); genes encoding expansin (expansin); and genes encoding ROS (peroxidase). More importantly, several corresponding proteins were significantly upregulated at the proteomic level, and the activation of the genes provided assurance for endosperm to break through the endosperm cell wall, in turn allowing smooth eelgrass seed germination. In addition to CWLFs, some cell wall remodeling enzymes (CWREs), associated with cell wall synthesis, loosening and enhancement, played indispensable roles in seed germination. CWREs generally include the same genes as the enzymatic CWLFs. For example, XTHs, which can also act as cell wall-modifying proteins, are involved in the regulation of embryonic axis or radicle elongation during seed germination [77, 78]. Our findings revealed that the gradual exposure of the cotyledon, as the seed germination process proceeded, implied a continuous process of cell wall degradation, modification, and remodeling, which was reflected in the heat map showing a substantial increase in the number and abundance of the associated genes.
Activation of antioxidant system
Studies have shown that when resting dry seeds absorb water, their oxygen uptake increased and mitochondrial energy metabolism was reactivated, which provided an important source of ROS [79]. Our analysis revealed that most of the DEGs and proteins identified in both stages were peroxidases, implying that peroxidases may be the main antioxidant enzymes for scavenging ROS in eelgrass during germination. In addition, we observed that lipoxygenase (LOX) as well as peroxiredoxins were important at both the transcriptome and proteome levels. LOX is widely believed to be involved in lipid mobilization during the early stages of seed germination, and facilitates ROS removal during the rapid mobilization of germinating seed reserves to alleviate the oxidative stress [56]. Peroxiredoxin is also a ROS scavenger that protects functional proteins from ROS during seed germination [52, 56]. Based on the heat map analysis, the number of up-regulated genes was greater in germination stage than in pre-germination stage, implying that the mitochondrial metabolic activity was enhanced during the germination process, resulting in greater ROS production.
HUB genes of the germination process
The results of PPI analysis of DEGs-DEPs at the pre-germination stage showed that the HUB gene at the center of the network was UDP-glucose 6-dehydrogenase (UGDH, Zosma01g01970), α-amylase, sucrose synthase, and s-adenosylmethionine synthase also have higher degree. However, at the germination stage, PPI analysis did not display a good aggregation result and the genes were dispersed. In plants, UGDH is one of the key enzymes involved in amino sugar and nucleotide sugar metabolism and is closely related to polysaccharide biosynthesis [80, 81]. Both amylase and sucrose synthase were significantly up-regulated at the transcriptional and protein levels, suggesting an important role during eelgrass seed germination. Many studies have also shown that starch hydrolases and sugar hydrolases were vital enzymes in the seed germination process [52, 82]. We think that during the germination phase of eelgrass seeds, under the action of amylase and sucrose synthase, the starch stored in the seeds begins to break down and produce ATP and carbohydrate material, which are used not only in seed germination but also in subsequent emergence of the radicle. Methionine metabolism also plays a major role in seed germination [83]. S-adenosylmethionine synthetase (metK) is a key enzyme involved in the synthesis of s-adenosylmethionine (AdoMet), which is a precursor of polyamine, vitamin biotin, and ethylene biosynthesis and provides essential metabolites for DNA synthesis, methylation regulation, and hormone regulation [27]. Our results revealed significant upregulation of metK at both the transcriptome and proteome levels, which is consistent with the characteristic accumulation of metK prior to radicle emergence [83, 84].
ERF transcription factors in eelgrass seed germination
Our analysis revealed that the ERF family was the most abundant in both stages, and presumably ERF TFs played an important role in the seed germination process. The AP2/ERF (APETALA2/ethylene response factor) structural domain family is a plant-specific [85, 86]. In recent years, several studies have revealed the regulatory mechanism of AP2/ERF-like TFs involved in plant seed germination. Liu and Wu [87] found that an ERF transcription factor isolated from tomato significantly reduced the sensitivity to ABA and auxin during seed germination by inhibiting a key component of the ABA signaling pathway, thereby promoting germination. The following year, Liu et al. [88] also found that ERF transcription factors could promote tomato seed germination through the GA-mediated glucose signaling pathway. Gupta et al. [85] also identified an AP2/ERF TF that negatively regulated ABA responses by altering ABA levels/signaling pathways, thereby promoting seed germination.
Conclusion
In the present study, transcriptomics and proteomics were combined to analyze the regulatory network and the dynamics of key physiological processes in eelgrass seeds at three stages from dormancy to germination under low salinity stimulation (Fig. 10). According to our results, low salinity stimulated the activation Ca2+ signaling and phosphatidylinositol signaling, and further initiated various subsequent germination-related physiological processes through the MAPK cascade. Starch, lipids, and storage proteins were actively mobilized to provide energy and substrate for germination; ABA synthesis and signal transduction were inhibited, whereas GA synthesis and signal transduction were activated, weakening seed dormancy and preparing seeds for germination; cell wall weakening and remodeling processes were activated to provide protection for cotyledon emergence. In addition, multiple antioxidant systems were activated to alleviate the oxidative stress generated during the germination process. PPI analysis of DEGs-DEPs of pre-germination revealed that HUB gene at the center of the network was udp-glucose 6-dehydrogenase (UGDH, Zosma01g01970), followed by α-amylase, sucrose synthase, suggesting that the activation of carbohydrate metabolism, such as starch, was essential to support eelgrass seed germination in the early stages. Identification of transcription factors revealed the highest number of ERF TFs in both stages, and it was hypothesized that the ERF family played an active role in the seed germination process. The present study investigated the mechanisms of eelgrass seed germination stimulated by low salinity, and further comprehensively analyzed the dynamics of key physiological processes at the transcriptome and proteome levels, providing experimental data for subsequent in-depth studies of the regulatory mechanisms related to seagrass seed germination.
Materials and methods
Experimental design
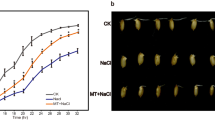
In this study, eelgrass seeds were collected from their natural habitats, and the collection processes of both conformed to local and national regulations. The voucher specimens of Z. marina were deposited in Marine Biological Museum of the Chinese Academy of Sciences (MBMCAS). The samples were identified by Yi Zhou, a Professor at IOCAS. Specifically, mature eelgrass seeds collected from Swan Lake, Weihai City, Shandong Province, China, in September 2021, and the seed germination season in the area was the following spring [89]. Collected seeds were stored in recirculating seawater tanks (1 m × 1.2 m × 1.5 m) in a laboratory. In December, the seeds are dormant and do not germinate under natural seawater conditions; however, low salinity conditions can promote rapid seed germination. Consequently, in December, 2,000 seeds (four replicates, 500 seeds per replicate) were placed in low salinity seawater with a salinity of 5 ppt; another 1,000 seeds (four replicates, 250 seeds per replicate) were placed in natural seawater with a salinity of 30 ppt. Seeds under both treatments were placed at a temperature of 15 °C and in the dark for germination. After 24 h, dormant seeds (DoS) were collected from the natural seawater group; dehiscent seeds (DeS) and germinated seeds (GeS) were collected from the low salinity seawater group (Fig. 1). The samples were immediately snap-frozen in liquid nitrogen for 15 min after collection, and then stored in a -80 °C refrigerator until the subsequent transcriptomic and proteomic analyses.
RNA sequencing and RT-qPCR
Seeds from three states (DoS/DeS/GeS, four replicates) were obtained for transcriptome sequencing, and the procedure was as follows: first, using TRIzol® Reagent to extract total RNA from eelgrass seed tissues, then DNase I (TaKara) were used to remove genomic DNA. We select high quality RNA samples to construct subsequent sequencing library. Secondly, we isolated mRNA, and further fragmented it with fragmentation buffer. Then, mRNA was used as templates to synthesize double-stranded cDNA. Subsequently, we performed end-repair, phosphorylation, and “A” base addition to synthesized cDNA, in which the target fragments of 300 bp were selected for libraries, which were then performed for 15 PCR cycles amplification on 2% Low Range Ultra Agarose. Finally, after quantification by TBS380, the Illumina NovaSeq 6000 sequencer (2 × 150-bp read length) were used to sequence the paired-end RNA-seq sequencing library.
To obtain clean reads with high quality, we performed trim and quality control on raw paired-end reads. Afterward, Zostera marina were selected as the reference genome for separately alignment of clean reads to get mapped reads. To identify DEGs (differentially expressed genes) between two different samples, the expression levels of each transcript were calculated according to the transcripts per million reads (TPM) method. RSEM was used to quantify gene abundances. DESeq2 software was used for differential expression analysis. Screening criteria for DEGs were: p-adjust < 0.05 and |log2FC| > 1.
Eight genes were selected for RT-qPCR to validate the transcriptome, with 18 S rRNA as reference gene (Table S5). The above extracted RNA was used as a template for reverse transcription to obtain cDNA. Pre-experimental results showed that the electrophoresis gel of each primer was a single bright band under specific conditions, indicating that there was no specific amplification and the primers were qualified. The RT-qPCR solution included 10 µL of 2× ChamQ SYBR Color qPCR Master Mix, 0.8 µL of both forward and reverse primers (5 μm each), 0.4 µL of 50× ROX Reference Dye, 2 µL of template (cDNA), and 6 µL of ddH2O, made-up to a total volume of 20 µL. Cycling conditions were as follows: the initial step was 95 °C for 5 min, and then 40 cycles (melting at 95 °C for 5 s, annealing at 55 °C for 30 s, and extension at 72 °C for 40 s). Each treatment group had three replicates, and each replicate sample had three multiple pores. The relative expression level was calculated using the 2−ΔΔCt method.
Proteome analyses
The 4D-label free quantitative proteomic analysis was performed on the same batch of samples (DoS1/2/3/4, DeS1/2/3/4, and GeS1/2/3/4) used for transcription profiling. The detailed operation was as follows: first, the total protein was extracted from the sample; secondly, the concentration of protein supernatant was determined using the Bicinchoninic acid (BCA) method; third, protein samples were subjected to SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel) electrophoresis analysis to evaluate whether the sample quality met the standards; fourth, the qualified protein samples were treated with reductive alkylation; fifth, an equal amount of protein from each sample was digested with trypsin; sixth, the peptides were desalted and peptide concentrations were determined; seventh, trypsin-digested peptides were analyzed using an EASY nLC-1200 system (Thermo, USA) coupled with a timsTOF Pro2 mass spectrometer (Bruker, Germany) at Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China); eighth, MS/MS spectra were searched using MaxQuant v2.0.3.1 software against the protein database. Finally, bioinformatic analysis of proteomic data was performed using the Majorbio Cloud platform (https://cloud.majorbio.com).
P-values and Fold change (FC) for the proteins between the two groups were calculated using R package “t-test”. The thresholds of fold change (> 1.5 or < 0.67) and P-value < 0.05 were used to identify differentially expressed proteins (DEPs). Functional annotation of all identified proteins was performed using GO (http://geneontology.org/) and KEGG pathway (http://www.genome.jp/kegg/). DEPs were further used for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. Protein-protein interaction (PPI) analysis was performed using String v11.5 (https://string-db.org/).
Data Availability
Transcriptomic sequencing data are available through the NCBI Sequence Read Archive under the accession number PRJNA964694, and the mass spectrometry proteomics data have been deposited to the Proteome Xchange Consortium via the iProX partner repository with the dataset identifier PXD042350.
References
Cullen-Unsworth L, Unsworth R. Seagrass meadows, ecosystem services, and sustainability. Environment. 2013;55:14–27.
Nordlund LM, Unsworth RKF, Gullstrom M, Cullen-Unsworth LC. Global significance of seagrass fishery activity. Fish Fish. 2018;19(3):399–412.
de los Santos CB, Olive I, Moreira M, Silva A, Freitas C, Luna RA, Quental-Ferreira H, Martins M, Costa MM, Silva J, Cunha ME, Soares F, Pousao-Ferreira P, Santos R. Seagrass meadows improve inflowing water quality in aquaculture ponds. Aquaculture. 2020;528:9.
Neto NC, Pomeroy A, Lowe R, Ghisalberti M. Seagrass meadows reduce wind-wave driven sediment resuspension in a sheltered environment. Front Mar Sci. 2022;8:16.
Lima MDC, Ward RD, Joyce CB, Kauer K, Sepp K. Carbon stocks in southern England’s intertidal seagrass meadows. Estuar Coast Shelf Sci. 2022;275:9.
Xu SC, Zhou Y, Qiao YL, Yue SD, Zhang XM, Zhang Y, Liu MJ, Zhang YL, Zhang ZH. Seagrass restoration using seed ball burial in northern China. Restor Ecol. 2023;31(1):8.
Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci U S A. 2009;106(30):12377–81.
Zhou Y, Liu P, Liu BJ, Liu XJ, Zhang XM, Wang F, Yang HS. Restoring eelgrass (Zostera marina L.) habitats using a simple and effective transplanting technique. PLoS ONE. 2014;9(4):7.
Shafer D, Bergstrom P. An introduction to a special issue on large-scale submerged aquatic vegetation restoration research in the Chesapeake Bay: 2003–2008. Restor Ecol. 2010;18(4):481–9.
Paling EI, van Keulen M, Wheeler K, Phillips J, Dyhrberg R. Mechanical seagrass transplantation in Western Australia. Ecol Eng. 2001;16(3):331–9.
van Katwijk MM, Thorhaug A, Marba N, Orth RJ, Duarte CM, Kendrick GA, Althuizen IHJ, Balestri E, Bernard G, Cambridge ML, Cunha A, Durance C, Giesen W, Han QY, Hosokawa S, Kiswara W, Komatsu T, Lardicci C, Lee KS, Meinesz A, Nakaoka M, O’Brien KR, Paling EI, Pickerell C, Ransijn AMA, Verduin JJ. Global analysis of seagrass restoration: the importance of large-scale planting. J Appl Ecol. 2016;53(2):567–78.
Grafnings MLE, Heusinkveld JHT, Hoeijmakers DJJ, Smeele Q, Wiersema H, Zwarts M, van der Heide T, Govers LL. Optimizing seed injection as a seagrass restoration method. Restor Ecol. 2023;31(3):8.
Orth RJ, Moore KA, Marion SR, Wilcox DJ, Parrish DB. Seed addition facilitates eelgrass recovery in a coastal bay system. Mar Ecol Prog Ser. 2012;448:177–95.
Orth RJ, Lefcheck JS, McGlathery KS, Aoki L, Luckenbach MW, Moore KA, Oreska MPJ, Snyder R, Wilcox DJ, Lusk B. Restoration of seagrass habitat leads to rapid recovery of coastal ecosystem services. Sci Adv. 2020;6(41):9.
Olsen JL, Rouze P, Verhelst B, Lin YC, Bayer T, Collen J, Dattolo E, De Paoli E, Dittami S, Maumus F, Michel G, Kersting A, Lauritano C, Lohaus R, Topel M, Tonon T, Vanneste K, Amirebrahimi M, Brakel J, Bostrom C, Chovatia M, Grimwood J, Jenkins JW, Jueterbock A, Mraz A, Stam WT, Tice H, Bornberg-Bauer E, Green PJ, Pearson GA, Procaccini G, Duarte CM, Schmutz J, Reusch TBH, Van de Peer Y. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature. 2016;530(7590):331–5.
Green EP, Short FT, Frederick T. World Atlas of Seagrasses. California, USA: University of California Press; 2003.
Orth RJ, Harwell MC, Bailey EM, Bartholomew A, Jawad JT, Lombana AV, Moore KA, Rhode JM, Woods HE. A review of issues in seagrass seed dormancy and germination: implications for conservation and restoration. Mar Ecol Prog Ser. 2000;200:277–88.
Pan JH, Han HW, Jiang X, Zhang WF, Zhao N, Song SF, Li X, Li XJ. Desiccation, moisture content and germination of Zostera marina L. seed. Restor Ecol. 2012;20(3):311–4.
Xu S, Xu SC, Zhou Y, Gu RT, Zhang XM, Yue SD. Long-term seed storage for desiccation sensitive seeds in the marine foundation species Zostera marina L. (eelgrass). Glob Ecol Conserv. 2020;24:11.
Xu SC, Zhou Y, Wang PM, Wang F, Zhang XM, Gu RT. Salinity and temperature significantly influence seed germination, seedling establishment, and seedling growth of eelgrass Zostera marina L. PeerJ. 2016;4:21.
Ridler MS, Dent RC, Arrinton DA. Effects of two Hurricanes on Syringodium filiforme, manatee grass, within the Loxahatchee River Estuary, Southeast Florida. Estuar Coasts. 2006;29:1019–25.
Steward JS, Virnstein RW, Lasi MA, Morris LJ, Miller JD, Hall LM, Tweedale WA. The impacts of the 2004 Hurricanes on hydrology, water quality, and seagrass in the central Indian river lagoon, Florida. Estuar Coasts. 2006;29(6):954–65.
He DL, Han C, Yao JL, Shen SH, Yang PF. Constructing the metabolic and regulatory pathways in germinating rice seeds through proteomic approach. Proteomics. 2011;11(13):2693–713.
Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–66.
Xue XF, Jiao FC, Xu HC, Jiao QQ, Zhang X, Zhang Y, Du SY, ** MH, Wang AG, Chen JT, Wang M. The role of RNA-binding protein, microRNA and alternative splicing in seed germination: a field need to be discovered. BMC Plant Biol. 2021;21(1):11.
Nonogaki H, Bassel GW, Bewley JD. Germination-still a mystery. Plant Sci. 2010;179(6):574–81.
Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D. Seed germination and Vigor. Annu Rev Plant Biol. 2012;63:507–33.
Taylor NL. Studies of the development of Zostera marina L. Can J Botany. 1957.
Sugiura H, Hiroe Y, Suzuki T, Maegawa M. The carbohydrate catabolism of Zostera marina influenced by lower salinity during the pre-germination stage. Fish Sci. 2009;75(5):1205–17.
Ma ZH, Bykova NV, Igamberdiev AU. Cell signaling mechanisms and metabolic regulation of germination and dormancy in barley seeds. Crop J. 2017;5(6):459–77.
Hao YQ, Hong YC, Guo HM, Qin PY, Huang AN, Yang XS, Ren GX. Transcriptomic and metabolomic landscape of quinoa during seed germination. BMC Plant Biol. 2022;22(1):13.
Huang H, Moller IM, Song SQ. Proteomics of desiccation tolerance during development and germination of maize embryos. J Proteom. 2012;75(4):1247–62.
Trindade BMC, Reis RS, Vale EM, Santa-Catarina C, Silveira V. Proteomics analysis of the germinating seeds of Cariniana Legalis (Mart.) Kuntze (Meliaceae): an endangered species of the Brazilian Atlantic Rainforest. Braz J Bot. 2018;41(1):129–9.
Phillips RC, Grant WS, McRoy CP. Reproductive strategies of eelgrass (Zostera-marina L). Aquat Bot. 1983;16(1):1–20.
Tanner CE, Parham T. Growing Zostera marina (eelgrass) from seeds in land-based culture systems for use in restoration projects. Restor Ecol. 2010;18(4):527–37.
Liu YL, Zhang XL, Song W, Wang ZL. Artificial seed germination and seedling production of Zostera marina L. by salinity manipulation. Acta Oceanol Sin. 2016;35(8):99–105.
Omoarelojie LO, Kulkarni MG, Finnie JF, van Staden J. Smoke-derived cues in the regulation of seed germination: are Ca2+-dependent signals involved? Plant Growth Regul. 2022;97(2):343–55.
Verma G, Khan S, Agarwal SK, Sharma S. Role of apoplastic calcium during germination and initial stages of seedling establishment in Vigna radiata seeds. J Plant Physiol. 2019;236:66–73.
Zhang Q, van Wijk R, Shahbaz M, Roels W, Schooten Bv, Vermeer JEM, Zarza X, Guardia A, Scuffi D, Garcia-Mata C, Laha D, Williams P, Willems LAJ, Ligterink W, Hoffmann-Benning S, Gillaspy G, Schaaf G, Haring MA, Laxalt AM, Munnik T. Arabidopsis phospholipase C3 is involved in lateral root initiation and ABA responses in seed germination and stomatal closure. Plant Cell Physiol. 2018;59(3):469–86.
Kashem MA, Itoh K, Iwabuchi S, Hori H, Mitsui T. Possible involvement of phosphoinositide-Ca2+ signaling in the regulation of alpha-amylase expression and germination of rice seed (Oryza sativa L). Plant Cell Physiol. 2000;41(4):399–407.
Mishra NS, Tuteja R, Tuteja N. Signaling through MAP kinasenetworks inplants. Arch Biochem Biophys. 2006;452:55–68.
Taj G, Agarwal P, Grant M, Kumar A. MAPK machinery in plants recognition and response to different stresses through multiple signal transduction pathways. Plant Signal Behav. 2010;5(11):1370–8.
Sreenivasulu N, Usadel B, Winter A, Radchuk V, Scholz U, Stein N, Weschke W, Strickert M, Close TJ, Stitt M, Graner A, Wobus U. Barley grain maturation and germination: metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol. 2008;146(4):1738–58.
Uchida M, Miyoshi T, Kaneniwa M, Ishihara K, Nakashimada Y, Urano N. Production of 16.5% v/v ethanol from seagrass seeds. J Biosci Bioeng. 2014;118(6):646–50.
Delefosse M, Povidisa K, Poncet D, Kristensen E, Olesen B. Variation in size and chemical composition of seeds from the seagrass Zostera marina-ecological implications. Aquat Bot. 2016;131:7–14.
Howell KA, Narsai R, Carroll A, Ivanova A, Lohse M, Usadel B, Millar AH, Whelan J. Map** metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Physiol. 2009;149(2):961–80.
Chen L, Wu JE, Li ZM, Liu Q, Zhao X, Yang HS. Metabolomic analysis of energy regulated germination and sprouting of organic mung bean (Vigna radiata) using NMR spectroscopy. Food Chem. 2019;286:87–97.
Glaubitz U, Li X, Schaedel S, Erban A, Sulpice R, Kopka J, Hincha DK, Zuther E. Integrated analysis of rice transcriptomic and metabolomic responses to elevated night temperatures identifies sensitivity- and tolerance-related profiles. Plant Cell Environ. 2017;40(1):121–37.
Yang PF, Li XJ, Wang XQ, Chen H, Chen F, Shen SH. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics. 2007;7(18):3358–68.
Pujar A, Jaiswal P, Kellogg EA, Ilic K, Vincent L, Avraham S, Stevens P, Zapata F, Reiser L, Rhee SY, Sachs MM, Schaeffer M, Stein L, Ware D, McCouch S. Whole-plant growth stage ontology for angiosperms and its application in plant biology. Plant Physiol. 2006;142(2):414–28.
Barros M, Fleuri LF, Macedo GA. Seed lipases: sources, applications and properties – a review. Braz J Chem Eng. 2010;27(1):15–29.
Ghosh S, Pal A. Identification of differential proteins of mungbean cotyledons during seed germination: a proteomic approach. Acta Physiol Plant. 2012;34(6):2379–91.
Erbas S, Tonguc M, Karakurt Y, Sanli A. Mobilization of seed reserves during germination and early seedling growth of two sunflower cultivars. J Appl Bot Food Qual. 2016;89:6.
Rosental L, Nonogaki H, Fait A. Activation and regulation of primary metabolism during seed germination. Seed Sci Res. 2014;24(1):1–15.
Gu JW, Chao HB, Gan L, Guo LX, Zhang K, Li YH, Wang H, Raboanatahiry N, Li MT. Proteomic dissection of seed germination and seedling establishment in Brassica napus. Front Plant Sci. 2016;7:19.
Han C, Yin XJ, He DL, Yang PF. Analysis of proteome profile in germinating soybean seed, and its comparison with rice showing the styles of reserves mobilization in different crops. PLoS ONE. 2013;8(2):9.
Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol. 2008;59:387–415.
Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y. Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol. 2020;62(1):25–54.
Tan BC, Joseph LM, Deng WT, Liu LJ, Li QB, Cline K, McCarty DR. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35(1):44–56.
Lopez-Molina L, Mongrand B, McLachlin DT, Chait BT, Chua NH. ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J. 2002;32(3):317–28.
Finkelstein RR, Gampala SSL, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14:15–S45.
Nakashima K, Yamaguchi-Shinozaki K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013;32(7):959–70.
Rubio S, Rodrigues A, Saez A, Dizon MB, Galle A, Kim TH, Santiago J, Flexas J, Schroeder JI, Rodriguez PL. Triple loss of function of protein phosphatases type 2 C leads to partial constitutive response to endogenous abscisic acid. Plant Physiol. 2009;150(3):1345–55.
Ma Y. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324(5932):1266–6.
Jacobsen JV, Barrero JM, Hughes T, Julkowska M, Taylor JM, Xu Q, Gubler F. Roles for blue light, jasmonate and nitric oxide in the regulation of dormancy and germination in wheat grain (Triticum aestivum L). Planta. 2013;238(1):121–38.
Shu K, Liu XD, **e Q, He ZH. Two faces of one seed: hormonal regulation of dormancy and germination. Mol Plant. 2016;9(1):34–45.
Bewley JD, Black M, Seeds. Physiology of Development and Germination. New York: Plenum; 1994. p. 367.
Regnault T, Daviere JM, Heintz D, Lange T, Achard P. The gibberellin biosynthetic genes AtKAO1 and AtKAO2 have overlap** roles throughout Arabidopsis development. Plant J. 2014;80(3):462–74.
Chen Y, Hou MM, Liu LJ, Wu S, Shen Y, Ishiyama K, Kobayashi M, McCarty DR, Tan BC. The Maize DWARF1 encodes a gibberellin 3-Oxidase and is dual localized to the Nucleus and Cytosol(W). Plant Physiol. 2014;166(4):2028–U1267.
Rubinovich L, Weiss D. The Arabidopsis cysteine-rich protein GASA4 promotes GA responses and exhibits redox activity in bacteria and in planta. Plant J. 2010;64(6):1018–27.
Chandrasekaran U, Zhao XT, Luo XF, Wei SW, Shu K. Endosperm weakening: the gateway to a seed’s new life. Plant Physiol Biochem. 2022;178:31–9.
Leubner-Metzger G. Seed after-ripening and over-expression of class I beta-1,3-glucanase confer maternal effects on Tobacco testa rupture and dormancy release. Planta. 2002;215(6):959–68.
Iglesias-Fernandez R, Rodriguez-Gacio MC, Barrero-Sicilia C, Carbonero P, Matilla A. Three endo-beta-mannanase genes expressed in the micropylar endosperm and in the radicle influence germination of Arabidopsis thaliana seeds. Planta. 2011;233(1):25–36.
Xu P, Cai XT, Wang Y, **ng L, Chen Q, **ang CB. HDG11 upregulates cell-wall-loosening protein genes to promote root elongation in Arabidopsis. J Exp Bot. 2014;65(15):4285–95.
Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407(6802):321–6.
Muller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 2009;150(4):1855–65.
Chen F, Nonogaki H, Bradford KJ. A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. J Exp Bot. 2002;53(367):215–23.
Romo S, Jiménez T, Labrador E, Dopico B. The gene for a xyloglucan endotransglucosylase/hydrolase from Cicer arietinum is strongly expressed in elongating tissues. Plant Physiol Biochem. 2005;43:169–76.
Pergo EM, Ishii-Iwamoto EL. Changes in energy metabolism and antioxidant defense systems during seed germination of the weed species Ipomoea triloba L. and the responses to allelochemicals. J Chem Ecol. 2011;37(5):500–13.
Wang SQ, Wang B, Hua WP, Niu JF, Dang KK, Qiang Y, Wang ZZ. De novo assembly and analysis of polygonatum sibiricum transcriptome and identification of genes involved in polysaccharide biosynthesis. Int J Mol Sci. 2017;18(9):17.
Qin CX, Chen ZL, Wang M, Li AM, Liao F, Li YR, Wang MQ, Long MH, Lakshmanan P, Huang DL. Identification of proteins and metabolic networks associated with sucrose accumulation in sugarcane (Saccharum spp. interspecific hybrids). J Plant Interact. 2021;16(1):166–78.
Cheng XX, **ong F, Wang CJ, **e H, He S, Geng GH, Zhou Y. Seed reserve utilization and hydrolytic enzyme activities in germinating seeds of sweet corn. Pak J Bot. 2018;50(1):111–6.
Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D. Importance of methionine biosynthesis for Arabidopsis seed germination and seedling growth. Physiol Plant. 2002;116(2):238–47.
Galland M, Huguet R, Arc E, Cueff G, Job D, Rajjou L. Dynamic proteomics emphasizes the importance of selective mRNA translation and protein turnover during Arabidopsis seed germination. Mol Cell Proteomics. 2014;13(1):252–68.
Gupta A, Upadhyay RK, Prabhakar R, Tiwari N, Garg R, Sane VA, Sane AP. SlDREB3, a negative regulator of ABA responses, controls seed germination, fruit size and the onset of ripening in tomato. Plant Sci. 2022;319:13.
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta-Gene Regul Mech. 2012;1819(2):86–96.
Liu HZ, Wu W. Comparative transcriptome analysis reveals function of TERF1 in promoting seed germination. Physiol Mol Biol Plants. 2021;27(8):1659–74.
Liu HZ, Yuan L, Guo W, Wu W. Transcription factor TERF1 promotes seed germination under osmotic conditions by activating gibberellin acid signaling. Plant Sci. 2022;322:10.
Xu S, Wang P, Zhou Y, Zhang X, Gu R, Liu X, Liu B, Song X, Xu S, Yue S. New insights into different reproductive effort and sexual recruitment contribution between two geographic Zostera marina L. populations in temperate China. Front Plant Sci. 2018; 9.
Acknowledgements
None.
Funding
This work was supported by the National Key R&D Program of China (2022YFC3105403), the National Natural Science Foundation of China (No. 32270405/42206142/32000269), the Natural Science Foundation of Shandong Province (ZR2020QD106), and the Taishan Scholars Program (Distinguished Taishan Scholars).
Author information
Authors and Affiliations
Contributions
YZ1 and YZ2 designed the experiments and wrote the original draft. YZ1 and SY performed the experiment. ML and XW offered help in plant materials preparation. SX and XZ helped to revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We complied with all relevant institutional, national and international guidelines for the collection of Z. marina seed with permissions from Institute of Oceanology Chinese Academy of Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material: Table S1.
Upregulated Differentially Expressed Proteins (DEPs) identified in main carbohydrate metabolism pathways in pre-germination stage (DoS vs. DeS). Table S2. Upregulated Differentially Expressed Proteins (DEPs) identified in main carbohydrate metabolism and lipid metabolism pathways in the germination stage (DeS vs. GeS). Table S3. Differentially Expressed Genes (DEGs) and Differentially Expressed Proteins (DEPs) identified in plant hormone signal transduction pathway (map04075) in the pre-germination stage. Table S4. Differentially Expressed Genes (DEGs) and Differentially Expressed Proteins (DEPs) identified in plant hormone signal transduction pathway (map04075) in the germination stage. Table S5. Primer sequences of RT-qPCR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y., Yue, S., Liu, M. et al. Combined transcriptome and proteome analysis reveal the key physiological processes in seed germination stimulated by decreased salinity in the seagrass Zostera marina L.. BMC Plant Biol 23, 605 (2023). https://doi.org/10.1186/s12870-023-04616-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04616-x