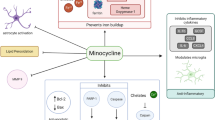
Abstract
TBI is a leading cause of death and disability in young people and older adults worldwide. There is no gold standard treatment for TBI besides surgical interventions and symptomatic relief. Post-injury infections, such as lower respiratory tract and surgical site infections or meningitis are frequent complications following TBI. Whether the use of preventive and/or symptomatic antibiotic therapy improves patient mortality and outcome is an ongoing matter of debate. In contrast, results from animal models of TBI suggest translational perspectives and support the hypothesis that antibiotics, independent of their anti-microbial activity, alleviate secondary injury and improve neurological outcomes. These beneficial effects were largely attributed to the inhibition of neuroinflammation and neuronal cell death. In this review, we briefly outline current treatment options, including antibiotic therapy, for patients with TBI. We then summarize the therapeutic effects of the most commonly tested antibiotics in TBI animal models, highlight studies identifying molecular targets of antibiotics, and discuss similarities and differences in their mechanistic modes of action.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability worldwide. Sixty-nine million individuals worldwide are estimated to sustain a TBI each year [1] and survivors are often left with enduring symptoms that can significantly affect their quality of life [2]. There are significant socio-economic consequences for TBI patients [3] and high medical costs associated with TBI [4, 5]. In Europe, TBI occurs most commonly due to falls, which has overtaken traffic accidents as the leading cause of injury in recent years [6, 7]. A bimodal age pattern (under 14 years old and older than 65 years) has been reported [8]. Young children aged 0–4 years are particularly vulnerable, with over 50,000 hospitalizations recorded annually in the US [9]. Due to the different mechanisms causing TBI, sex and age are unequally distributed. While falls are more common in older women, traffic accidents, violence and sports injuries are the predominant causes of TBI in young men. The overall proportion of men reported to suffer a TBI is always higher than that of woman [10], but functional outcomes do not differ significantly between men and woman [11]. However, women may be more susceptible to persistent cognitive symptoms after mild TBI than men [12].
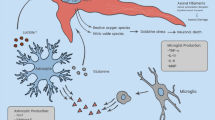
The pathophysiology of TBI is multifactorial and the primary injury triggers a complex cascade of events, including impaired cerebral autoregulation of perfusion, blood-brain barrier (BBB) leakage, edema formation, oxidative stress, disruption of Ca2+ homeostasis, and mitochondrial dysfunction [13,14,15,16]. These interconnected processes lead to neuronal cell death and long-lasting brain inflammatory responses that can aggravate a wide range of clinical conditions [17] and can also have significant effects on peripheral organs [18]. However, it has become clear that inflammation has both harmful but also beneficial effects on neuronal survival and brain repair, e.g. by promoting the clearance of cell debris [17, 19,20,21,22]. Recent findings also link dysfunction of the glymphatic-meningeal lymphatic system with secondary events after TBI, such as edema and increased intracranial pressure (ICP), brain inflammatory responses, and insufficient clearance of cell debris and toxic metabolites [23,24,25,26].
Given the great heterogeneity in etiology, presentation, pathophysiology, complexity, and outcome, the care for patients with TBI proves to be a challenge [27]. In fact, the complexity of TBI pathophysiology limits the possibility for a general or causal therapeutic approach and the unpredictability of the individual pathophysiology requires close monitoring of the injured brain to tailor the treatment to the patient`s specific status [14].
Antibiotics are often part of the treatment of TBI patients. They can prevent or cure infections that can occur in open head injury patients, after neurosurgical interventions, or in mechanically ventilated patients (see studies listed in Table 1). Antibiotics can also have therapeutic effects independent of their anti-microbial activity, as shown by studies in TBI animal models (see studies listed in Table 2). In this review, we briefly summarize the current treatment options and clinical use of antibiotics in TBI. We further provide an overview of the therapeutic effects of the most commonly tested antibiotics in TBI animal models, highlight studies identifying molecular targets of antibiotics, and discuss similarities and differences in their mechanistic modes of action.
Current treatment options in human patients with TBI
Acute treatment options for patients with TBI encompass surgical interventions, such as evacuation of intracranial hematomas, repair of skull fractures, decompressive craniectomy, and supportive measures to relieve symptoms and maintain homeostasis.
In recent years, significant advances have been made in neuromonitoring, neuroimaging, and surgical interventions to maintain normocapnia, normoxia and normothermia, and to positively influence outcome [28,29,30]. However, surgical interventions are often ambiguous or fail to improve outcome with regard to surgical technique (e.g. craniotomy versus decompressive craniectomy) and timing [31,32,33,34,35]. Advances in neuromonitoring comprise, for example, the detailed analysis of ICP using machine learning models, which can improve the prediction of outcomes and mortality [36,37,38,39,40] It is likely that novel and improved neuromonitoring methods will have an increasing impact on treatment strategies [36, 41, 42].
It is important to note that rigorous treatment trials (e.g., randomized control trials) or meta-analyses are complicated by the absence of a standardized methodological approach and focus on individualized treatment strategies with frequent re-evaluation. Indeed, this could be a major reason for the failure of most pharmaceutical interventions in clinical trials. Nevertheless, some trials have opened up new therapeutic options for other diseases or expanded the possibilities for the application of neuroprotective drugs [43].
Antibiotic therapy in human patients with TBI
Post-injury infections are common in-hospital complications of TBI, affecting up to 55% of patients with moderate-severe TBI [18] and up to 75% of adult patients with severe TBI [44, 45]. The high susceptibility to infection has been associated with general immune suppression in TBI patients [44, 46]. The major infection types are those affecting the lower respiratory tract and surgical site [44], including nosocomial pneumonia after aspiration, device-related infections (e.g. ventilator-associated pneumonia or catheter-related infections), and meningitis following cranial procedures (e.g. surgery and/or device placement) [48,49,50,50]. A negative association between post-injury infections, clinical outcome or higher mortality rates was reported in TBI [51, 52]. Antibiotic treatment has been postulated to be relevant to the outcome of TBI patients [43] and early administration of antibiotics in TBI patients results in lower mortality rates [53]. Along this line, early antibiotic prophylaxis delayed, and prevented nosocomial infections and ventilation-associated pneumonia in patients with severe TBI but did not change length of hospital stay or mortality [54, 55]. Positive clinical effects, albeit not reaching a statistically significant level, were reported in a meta-analysis on antibiotic prophylaxis for ventricular drain placement in patients with basal skull fractures [56]. To the best of our knowledge, there is currently no general evidence-based guideline for the administration of antibiotics in TBI patients.
Currently, a wide range of antibiotics is used for anti-infectious treatment in TBI patients. The choice of antibiotics may depend not only on the patient`s health status and the diversity of pathogenic bacteria classes infecting the patient, but also on hospital-specific guidelines and physician-specific preferences. Nevertheless, some recommendations and anti-infective regimens have been published for antibiotic treatment of patients with open or penetrating TBI. The predominating strategy is early intravenous administration of antibiotics that cover the spectrum of gram-positive and gram-negative bacteria (e.g. amoxicillin/clavulanic acid, or 2nd or 3rd generation cephalosporins, such as cefazolin, ceftriaxone, or cefuroxime), optionally supplemented with an aminoglycoside and vancomycin, and metronidazole or meropenem if anaerobic infection is suspected [50, 61,62,63,64,65]. To our knowledge, there are no direct studies on the sex-specific effects of antibiotics and no sex-specific recommendations regarding the type of antibiotics.
It should be noted that prolonged prophylactic antibiotic use (> 48 h) for nosocomial pneumonia in trauma patients has been reported to carry the risk of antibiotic complications and a higher incidence of multidrug-resistant bacteria [66]. Another possible caveat is that antibiotic therapy has a significant impact on the gut microbiome and can promote opportunistic pathogenic bacteria, such as Clostridioides difficile, potentially associated with severe symptoms in patients [67,68,69,70]. Colonization with multi-drug resistant bacteria was found in fecal samples from 25 of 39 TBI patients (64%) as early as 48 h after admission to an intensive care unit [71]. This supports the hypothesis that TBI associates with an increased ratio of pathogenic to commensal bacteria, as suggested in a recent systematic review that included an observatory study with human TBI patients and six animal studies [85]. Such a microbiome shift has also been observed in chronic TBI, potentially leading to long-lasting changes in bacterial nutrient utilization, which may contribute to reduced availability of amino acids in TBI patients [86, 87].
Altogether, infections are relevant complications in TBI patients and require symptomatic antibiotic therapy, whereas prophylactic antibiotic therapy appears to be rather empirical at present and possible negative effects, such as antibiotic-induced microbiome dysbiosis, must be taken into account. As the role of the gut-brain axis in TBI is beyond the scope of this review article, we would like to refer readers to excellent reviews that have focused on this exciting topic [88,89,90,91].
Antibiotics in animal models of TBI
In contrast to the small number of clinical studies, a larger number of experimental studies have examined the effects of different classes of antibiotics in animal models of TBI. Details of selected studies are shown in Table 2.
The models used comprise the controlled cortical impact (CCI) model, the lateral fluid percussion (LFP) model, and closed-head injury weight-drop models. It should be kept in mind that none of these injury models alone can model the wide spectrum of human TBI and that rodent models are dominating the research field [92, 93]. However, rodent models can shed light on pathophysiologically relevant processes also known to occur in human TBI patients. Importantly, they provide the possibility to perform standardized preclinical studies, thereby preventing uncontrolled experimental variabilities, which may add to the already highly heterogeneous etiology, presentation, and complexity of human TBI. However, it should be noted that most experimental studies used male mice and sex was not considered as a biological variable. This would require comparing male and female mice in the same study. Briefly summarized, experimental data on minocycline administered alone, or in combination with N-acetylcysteine, support the inhibition of microglia activation, associated neuroinflammation, and anti-apoptotic effects as the principal mechanism of action [78,79,80,81]. Two experimental TBI studies considered sex as a biological variable [78, 79]. Taylor et al. reported short-term anti-pyretic effects of minocycline irrespective of sex and increased IL-1β and IL-6 levels in the hippocampus of minocycline-treated ovariectomized female rats, but not in male rats [78]. A comprehensive study by Celorrio et al. showed inhibition of injury-induced microglia activation and neurodegeneration in the hippocampus as well as long-term improved fear memory performance and increased synapse densities following minocycline treatment without significant sex-differences [79]. Doxycycline also showed anti-inflammatory and anti-apoptotic effects [83], BBB-protective effects [84], and improved outcomes [83, 94]. Ceftriaxone was found be neuroprotective, which has been associated with the up-regulation of the glutamate transporter GLT-1 (also termed EAAT2, encoded by the SLC1A2 gene) [72, 94,95,96]. Trovafloxacin, showed anti-inflammatory and neuroprotective actions attributed to the inhibition of the pannexin channel Panx1 [76].
Consistent with observations in TBI patients, several animal studies provided evidence that TBI induces rapid and long-lasting changes in gut microbiota and metabolites, which in turn may influence TBI pathogenesis [71, 86, 97,98,99,100,101,102,103]. For example, gut microbiome dysbiosis has been associated with impaired neurogenesis and increased pro-inflammatory activation of microglia in a mouse model of TBI [102, 103] and alterations in the gut microbiome were found to influence the permeability of the BBB [104], which is a key structure in TBI pathogenesis. Adverse effects of bacterial metabolites produced under pathogenic conditions may account for these observations [89, 105]. These results from animal studies support the above-mentioned safety considerations for the use of antibiotics in human TBI patients with regard to possible adverse effects related to changes in the microbiome.
In the following sections, the effects and mechanisms of commonly used antibiotics in rodent models of TBI are reviewed and discussed in light of existing data on human TBI.
Minocycline
Minocycline, a tetracycline derivative, is considered the most effective at providing neuroprotection [106,107,108]. There is a substantial number of therapeutic studies in animal models of TBI that demonstrate its neuroprotective effects (examples in Table 2) [77, 79, 81, 109,110,111,112,113,114,115,116,117,118], including synergistic effects with N-acetylcysteine [80, 119,120,121,122]. The experimental data support the inhibition of microglia activation and associated neuroinflammation as the principal mechanism of action. Several properties of minocycline may contribute to these effects. In general, tetracyclines including minocycline form poorly soluble chelates with bivalent and trivalent cations, particularly calcium, magnesium, aluminium, and iron [123]. This property may be related to the anti-oxidative and neuroprotective effects of minocycline. Indeed, biochemical in vitro data support anti-oxidative actions of minocycline, showing a Fe2+- and Fe3+- chelating activity [124] and a scavenger function for peroxynitrite [125]. Minocycline also attenuated iron neurotoxicity in cultures of primary mouse neurons [126] and after experimental intracerebral hemorrhage [127]. In addition, minocycline may mitigate the iron-induced pro-inflammatory activation of microglia and macrophages following the uptake of erythrocytes, hemoglobin, and heme after CNS injury [128].
Other work suggested that minocycline inhibits the release of cytochrome c from mitochondria, a key step during the early phase of apoptosis [129], which may contribute to neuroprotective effects independent from anti-inflammatory actions [130]. Finally, indirect evidence in a murine closed-head trauma model suggested that cannabinoid receptors are required for neuroprotective actions of minocycline because protective actions of minocycline were prevented by cannabinoid receptor antagonists [131].
Collectively, the evidence for minocycline can explain a wide spectrum of treatment effects. The positive pleiotropic effects in experimental models as well as its safety profile and effective crossing of the BBB suggest that minocycline is particularly suitable for the treatment of patients after TBI [132]. However, the results of some experimental TBI studies do not support sustained neuroprotection or improved behavioral outcomes after treatment with minocycline [133,134,135,136].
Nevertheless, the promising results from preclinical studies prompted first clinical trials, however the results on the neuroprotective benefits of minocycline remain controversial [57, 58, 137, 138]. It has been concluded that minocycline shows potential efficacy in treating acute but not chronic TBI [121]. In fact, minocycline administration for 12 weeks caused reduction of microgliosis in chronic TBI patients. However, this effect coincided with increased plasma levels of neurofilament light chain (NF-L), a biomarker for axonal damage, which has led to the conclusion that minocycline increased neurodegeneration [57]. The authors discussed that chronically activated microglia may have trophic rather than deleterious effects, consistent with earlier results in rhesus monkey [139, 140]. In support of this explanation, Safaiyan et al. identified a population of white-matter associated microglia with a potentially protective role during white matter aging and disease [141]. An alternative, albeit speculative, explanation would be that increased plasma levels of NF-L were caused by minocycline-mediated reduction of microglia with high phagocytic activity and not by neurodegeneration.
However, up until now, most of the human trials could be considered pilot studies. Prospective, randomized multicenter trials, particularly those focusing on optimal dosing and timing, are needed [142]. Moreover, human clinical trials investigating the potentially synergistic combination of minocycline with N-acetylcysteine are warranted [143].
Doxycycline
Doxycycline is another antibiotic of the tetracycline class that has been investigated in TBI animal models (Table 2). In general, tetracyclines such as doxycycline as well as minocycline show iron-chelating activity [123].
Recent studies showed that doxycycline attenuates BBB leakage and vascular hyperpermeability [84], suppresses neuroinflammation and apoptosis [82], and improves neurological outcomes [83, 94]. Similar to minocycline, the iron-chelating effects of doxycycline may contribute to its neuroprotective effects, as excess iron can damage the injured brain via ferroptosis or other iron-mediated mechanisms [144, 145]. Indeed, doxycycline protects neurons from iron-dependent ferroptosis in vitro [146].
Important molecular insights were gained by combining in silico molecular docking studies and enzymatic activity assays in brain tissue preparations after murine TBI. Evidence was provided that doxycycline binds to the active site of the proteolytic enzyme matrix metalloproteinase 9 (MMP-9) [84] and decreases MMP-9 serum levels after experimental TBI in rats [147]. These results have potential clinical relevance because MMP-9 is associated with BBB disruption and secondary hemorrhage [148] and may serve as an outcome-relevant CSF biomarker along with other MMPs in TBI patients [149]. The underlying molecular mechanisms are diverse due to the broad substrate specificity of MMP-9, as demonstrated by secretome analysis in murine experimental autoimmune encephalomyelitis, which uncovered 119 potential MMP-9 substrates [150]. Interestingly, experimental stroke studies using gene-deficient mice provided indirect evidence that MMP-9 is also a possible target of minocycline [151], and minocycline also exhibits iron-chelating activity similar to doxycycline [123], suggesting common mechanisms of action.
Thus, doxycycline may interfere with iron-mediated deleterious processes and inhibit MMP-9, a relevant pathophysiological molecular target. Given clinical findings of reduced serum levels of the neuronal injury marker NSE after doxycycline treatment [59], rigorous testing of doxycycline in prospective clinical trials would be important.
Ceftriaxone
Ceftriaxone is a β-lactam antibiotic of the cephalosporin group. Neuroprotective effects of ceftriaxone were shown after experimental TBI in rats (Table 2). Posttraumatic administration of ceftriaxone reduced brain edema, pro-inflammatory cytokine expression, and cognitive deficits. Moreover, restored protein expression levels of the glutamate transporter GLT-1 (also termed EAAT2, encoded by the SLC1A2 gene) were associated with the neuroprotective effects of ceftriaxone [74]. Subsequent studies confirmed the association between ceftriaxone, GLT-1, and neuroprotection [94,95,96]. Moreover, repeated administration of ceftriaxone attenuated neuronal apoptosis [96, 152] and neuronal autophagy [95], and TBI-induced astrogliosis and seizures [75]. The findings also suggested that ceftriaxone-mediated restoration of GLT-1 expression attenuates glutamate excitotoxicity. In support of this hypothesis, it was demonstrated that ceftriaxone protects cortical parvalbumin-positive interneurons susceptible from excitotoxicity, thereby attenuating TBI-induced loss of intracortical inhibition [72]. More recent data demonstrated that administration of ceftriaxone during the chronic phase of experimental TBI reduces seizure susceptibility to the convulsant pentylenetetrazol, possibly due to augmented expression levels of the glutamate transporters GLT-1 and GLAST- 1 (also termed EAAT1, encoded by the SCL1A3 gene) [153].
There is evidence for a molecular target of ceftriaxone. Given the crucial role of the nuclear factor kappa-B (NF-kB) signaling pathway for GLT-1/EAAT2 induction by ceftriaxone [152, 154], a recent study addressed the question of how ceftriaxone triggers transcriptional activity of NF-kB without increasing pro-inflammatory gene expression. Using in silico 3D homology modelling, a possible explanation was provided by predicting ceftriaxone binding sites and its conformational effects on NF-kB´s site-specific DNA interaction responsible for EAAT-2 expression [155]. Some questions remain about the precise molecular mechanisms and molecular targets of ceftriaxone. The view that extracellular glutamate is an exclusive trigger of excitotoxicity is not without controversy [156,157,158], and glutamate biosensor imaging studies indicated that ceftriaxone decreased glutamate release independent of GLT-1 expression levels [159].
Altogether, experimental data support neuroprotective effects of ceftriaxone. The underlying mechanisms are still elusive, but some evidence point to a crucial role of the transcription factor NF-kB and the release and uptake regulation of the excitatory neurotransmitter glutamate.
Trovafloxacin
Trovafloxacin is a broad-spectrum fluoroquinolone-derived antibiotic. Treatment studies using a murine CCI model of TBI showed that trovafloxacin has neuroprotective and anti-inflammatory effects [76]. On the other hand, structural similarities of fluoroquinolones to endogenous glutamate receptor ligands, such as kynurenic acid, suggest an interaction with ligand-gated glutamate receptors. Indeed, trovafloxacin, along with other fluoroquinolones, evoked excitatory responses in acute hippocampal slice preparations from rats [160]. Moreover, trovafloxacin has been found to be associated with adverse CNS events [161]. Later studies identified trovafloxacin as an inhibitor of pannexin-1 (Panx-1) channels [162]. These channels mediate paracrine and autocrine signaling via ions and small metabolites including Ca2+ and ATP [163], thereby serving as sensors and effectors for changes in the cellular microenvironment [164]. Panx-1-mediated ATP release has been associated with pathogenic processes in TBI, such as inflammasome activation and pyroptosis, immune cell infiltration as well as glia proliferation and scar formation [165, 166]. Panx-1 is expressed in blood cells, immune cells, neurons and glia (www.proteinatlas.org). However, subjecting myeloid-specific Panx-1 knockout mice to the CCI model of TBI revealed that Panx-1 mediates the infiltration of peripheral immune cells, leukocytes, macrophages, and neutrophils, whereas the number of brain-resident microglia was not affected by myeloid-specific Panx-1-deficiency. Interestingly, both MMP-9 levels and spectrin breakdown products (SBDPs), a marker of axonal injury and excitotoxicity in the CCI model of TBI [167], were attenuated in myeloid-specific Panx-1 knockout mice [168].
Overall, several studies indicate potent effects of trovafloxacin in the context of neuronal excitation as well as brain inflammation. It remains to be clarified whether these effects are equally relevant after TBI and whether they are synergistic or opposing in terms of neuroprotective and anti-inflammatory actions.
Other antibiotics
This review focuses on the most commonly investigated antibiotics in experimental TBI. There are only a few studies on other antibiotics in brain injury models. For example, ampicillin has been shown to increase the expression of the glutamate transporter GLT-1 and to reduce neuronal damage in a rodent model of transient forebrain ischemia [169]. This suggests a similar mode of action to ceftriaxone, but data on experimental TBI are lacking. Ampicillin has also been combined with metronidazole, neomycin and vancomycin to achieve depletion of gut microbiota in experimental TBI [170]. However, this study did not aim to reveal neuroprotective effects of antibiotics independent of their anti-microbial activity. Vancomycin, a bactericide glycopeptide antibiotic used primarily for the spectrum of gram-positive infections, mediated BBB-related neuroprotection in a Staphylococcus epidermis-potentiated model of neonatal hypoxic-ischemic brain injury. However, these observations may be due to vancomycin`s anti-infective rather than neuroprotective effect [171]. Rifampicin, an effective antibiotic for the treatment of tuberculosis, showed neuroprotective effects and improved cognitive impairment after global cerebral ischemia in rats, which has been attributed to the activation of the pathophysiologically relevant transcription factor NRF2 [172]. Rifampicin further inhibits neuronal cell death via inhibition of microglia activation in vitro [173]. It has been proposed to reduce neurotoxicity of amyloid beta protein through an antioxidative mechanism [174], and therefore could be a promising test candidate for experimental TBI. In summary, some data on the antibiotics ampicillin, vancomycin, and rifampicin suggest neuroprotective potential and further studies are needed to test their potential clinical relevance.
Conclusions
There is an unmet need for clinical therapeutic options in the treatment of TBI. Increasing evidence suggests that antibiotic therapies may have neuroprotective and anti-inflammatory effects besides their anti-microbial mechanisms of action. However, the evidence for prophylactic antibiotic therapy is somewhat empirical at present and evidence-based standard operating procedures for the prophylactic use of antibiotics need to be established. The benefit of antibiotic therapy should be carefully considered in the light of adverse effects on the gut microbiome and the gut-brain axis. Some evidence from experimental research points to a mechanistic overlap of neuroprotective and anti-inflammatory actions of antibiotics. At least between minocycline and doxycycline, there are mechanistic similarities in terms of antioxidant and iron-chelating activities, and molecular targets, such as MMP-9. Further identification and characterization of molecular targets is of paramount importance to demonstrate the relevance of standardized antibiotic-based therapies in a variety of animal models of TBI across different laboratories.
Data availability
Not applicable.
References
Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018:1–18.
Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care (London England). 2016;20(1):148.
Norup A, Kruse M, Soendergaard PL, Rasmussen KW, Biering-Sørensen F. Socioeconomic consequences of traumatic Brain Injury: a Danish Nationwide Register-based study. J Neurotrauma. 2020;37(24):2694–702.
Miller GF, DePadilla L, Xu L. Costs of Nonfatal Traumatic Brain Injury in the United States, 2016. Med Care. 2021;59(5):451–5.
Leibson CL, Brown AW, Hall Long K, Ransom JE, Mandrekar J, Osler TM, et al. Medical care costs associated with traumatic brain injury over the full spectrum of disease: a controlled population-based study. J Neurotrauma. 2012;29(11):2038–49.
Peeters W, van den Brande R, Polinder S, Brazinova A, Steyerberg EW, Lingsma HF, et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015;157(10):1683–96.
Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Reviews Neurol. 2013;9(4):231–6.
Gardner AJ, Zafonte R. Neuroepidemiology of traumatic brain injury. Handb Clin Neurol. 2016;138:207–23.
Yue JK, Upadhyayula PS, Avalos LN, Cage TA. Pediatric Traumatic Brain Injury in the United States: rural-urban disparities and considerations. Brain Sci. 2020;10(3).
Brazinova A, Rehorcikova V, Taylor MS, Buckova V, Majdan M, Psota M et al. Epidemiology of traumatic brain Injury in Europe: a living systematic review. J Neurotrauma. 2018.
Gupte R, Brooks W, Vukas R, Pierce J, Harris J. Sex differences in traumatic Brain Injury: what we know and what we should know. J Neurotrauma. 2019;36(22):3063–91.
Levin HS, Temkin NR, Barber J, Nelson LD, Robertson C, Brennan J, et al. Association of sex and age with mild traumatic Brain Injury-related symptoms: a TRACK-TBI study. JAMA Netw Open. 2021;4(4):e213046.
Schäfer MK, Pfeiffer A, Jaeckel M, Pouya A, Dolga AM, Methner A. Regulators of mitochondrial ca(2+) homeostasis in cerebral ischemia. Cell Tissue Res. 2014;357(2):395–405.
Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9.
Cash A, Theus MH. Mechanisms of blood-brain barrier dysfunction in traumatic brain Injury. Int J Mol Sci. 2020;21(9).
Dietvorst S, Depreitere B, Meyfroidt G. Beyond intracranial pressure: monitoring cerebral perfusion and autoregulation in severe traumatic brain injury. Curr Opin Crit Care. 2023;29(2):85–8.
Morganti-Kossmann MC, Semple BD, Hellewell SC, Bye N, Ziebell JM. The complexity of neuroinflammation consequent to traumatic brain injury: from research evidence to potential treatments. Acta Neuropathol. 2019;137(5):731–55.
McDonald SJ, Sharkey JM, Sun M, Kaukas LM, Shultz SR, Turner RJ, et al. Beyond the brain: peripheral interactions after traumatic brain Injury. J Neurotrauma. 2020;37(5):770–81.
Alam A, Thelin EP, Tajsic T, Khan DZ, Khellaf A, Patani R, et al. Cellular infiltration in traumatic brain injury. J Neuroinflammation. 2020;17(1):328.
Wang Y, Wernersbach I, Strehle J, Li S, Appel D, Klein M, et al. Early posttraumatic CSF1R inhibition via PLX3397 leads to time- and sex-dependent effects on inflammation and neuronal maintenance after traumatic brain injury in mice. Brain Behav Immun. 2022;106:49–66.
Schäfer MKE, Tegeder I. NG2/CSPG4 and progranulin in the posttraumatic glial scar. Matrix Biol. 2018;68–9:571–88.
Simon DW, McGeachy MJ, Bayır H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Reviews Neurol. 2017;13(3):171–91.
Bolte AC, Lukens JR. Neuroimmune cleanup crews in brain injury. Trends Immunol. 2021;42(6):480–94.
Bolte AC, Dutta AB, Hurt ME, Smirnov I, Kovacs MA, McKee CA, et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun. 2020;11(1):4524.
Liao J, Zhang M, Shi Z, Lu H, Wang L, Fan W, et al. Improving the function of meningeal lymphatic vessels to promote brain edema absorption after traumatic brain Injury. J Neurotrauma. 2023;40(3–4):383–94.
Hussain R, Tithof J, Wang W, Cheetham-West A, Song W, Peng W et al. Potentiating glymphatic drainage minimizes post-traumatic cerebral oedema. Nature. 2023.
Fong R, Konakondla S, Schirmer CM, Lacroix M. Surgical interventions for severe traumatic brain injury. J Emerg Crit Care Med. 2017;1(10).
Hays LMC, Udy A, Adamides AA, Anstey JR, Bailey M, Bellapart J, et al. Effects of brain tissue oxygen (PbtO(2)) guided management on patient outcomes following severe traumatic brain injury: a systematic review and meta-analysis. J Clin Neuroscience: Official J Neurosurgical Soc Australasia. 2022;99:349–58.
Tas J, Czosnyka M, van der Horst ICC, Park S, van Heugten C, Sekhon M, et al. Cerebral multimodality monitoring in adult neurocritical care patients with acute brain injury: a narrative review. Front Physiol. 2022;13:1071161.
Lazaridis C, Foreman B. Management strategies based on Multi-modality Neuromonitoring in severe traumatic brain Injury. Neurotherapeutics. 2023;20(6):1457–71.
van Essen TA, Lingsma HF, Pisică D, Singh RD, Volovici V, den Boogert HF, et al. Surgery versus conservative treatment for traumatic acute subdural haematoma: a prospective, multicentre, observational, comparative effectiveness study. Lancet Neurol. 2022;21(7):620–31.
van Essen TA, van Erp IAM, Lingsma HF, Pisică D, Yue JK, Singh RD, et al. Comparative effectiveness of decompressive craniectomy versus craniotomy for traumatic acute subdural hematoma (CENTER-TBI): an observational cohort study. EClinicalMedicine. 2023;63:102161.
Gantner D, Wiegers E, Bragge P, Finfer S, Delaney A, van Essen T, et al. Decompressive Craniectomy Practice following traumatic Brain Injury in comparison with randomized trials: Harmonized, Multi-center Cohort studies in Europe, the United Kingdom, and Australia. J Neurotrauma. 2022;39(11–12):860–9.
Shah A, Almenawer S, Hawryluk G. Timing of Decompressive Craniectomy for ischemic stroke and traumatic Brain Injury: a review. Front Neurol. 2019;10:11.
Kolias AG, Adams H, Timofeev I, Czosnyka M, Corteen EA, Pickard JD, et al. Decompressive craniectomy following traumatic brain injury: develo** the evidence base. Br J Neurosurg. 2016;30(2):246–50.
Jung MK, Ahn D, Park CM, Ha EJ, Roh TH, You NK et al. Prediction of Serious Intracranial Hypertension from Low-Resolution Neuromonitoring in Traumatic Brain Injury: an Explainable Machine Learning Approach. IEEE J Biomed Health Inf. 2023;Pp.
Carra G, Güiza F, Piper I, Citerio G, Maas A, Depreitere B, et al. Development and External Validation of a machine learning model for the early prediction of doses of harmful intracranial pressure in patients with severe traumatic brain Injury. J Neurotrauma. 2023;40(5–6):514–22.
Petrov D, Miranda SP, Balu R, Wathen C, Vaz A, Mohan V, et al. Prediction of intracranial pressure crises after severe traumatic brain injury using machine learning algorithms. J Neurosurg. 2023;139(2):528–35.
Kazimierska A, Uryga A, Mataczynski C, Burzynska M, Ziolkowski A, Rusiecki A, et al. Analysis of the shape of intracranial pressure pulse waveform in traumatic brain Injury patients. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:546–9.
Uryga A, Ziółkowski A, Kazimierska A, Pudełko A, Mataczyński C, Lang EW, et al. Analysis of intracranial pressure pulse waveform in traumatic brain injury patients: a CENTER-TBI study. J Neurosurg. 2023;139(1):201–11.
Park D, Kim I. Application of machine learning in the field of Intraoperative Neurophysiological Monitoring: a narrative review. Appl Sci. 2022;12(15):7943.
Al-Mufti F, Kim M, Dodson V, Sursal T, Bowers C, Cole C, et al. Machine learning and Artificial Intelligence in Neurocritical Care: a Specialty-wide disruptive Transformation or a strategy for success. Curr Neurol Neurosci Rep. 2019;19(11):89.
Zhao Q, Li H, Li H, Zhang J. Research progress on pleiotropic neuroprotective drugs for traumatic brain injury. Front Pharmacol. 2023;14:1185533.
Hazeldine J, Lord JM, Belli A. Traumatic brain Injury and Peripheral Immune suppression: primer and Prospectus. Front Neurol. 2015;6:235.
Pandya A, Chaput KH, Schertzer A, Moser D, Guilfoyle J, MacGillivray S, et al. Risk of infection and Sepsis in Pediatric patients with traumatic brain Injury admitted to Hospital following major trauma. Sci Rep. 2018;8(1):9798.
Sharma R, Shultz SR, Robinson MJ, Belli A, Hibbs ML, O’Brien TJ et al. Infections after a traumatic brain injury: The complex interplay between the immune and neurological systems. Brain, Behavior, and Immunity. 2019;79:63–74.
Hamele M, Stockmann C, Cirulis M, Riva-Cambrin J, Metzger R, Bennett TD, et al. Ventilator-Associated Pneumonia in Pediatric Traumatic Brain Injury. J Neurotrauma. 2016;33(9):832–9.
Kourbeti IS, Vakis AF, Papadakis JA, Karabetsos DA, Bertsias G, Filippou M, et al. Infections in traumatic brain injury patients. Clin Microbiol Infection: Official Publication Eur Soc Clin Microbiol Infect Dis. 2012;18(4):359–64.
Busl KM. Nosocomial infections in the Neurointensive Care Unit. Neurosurg Clin North Am. 2018;29(2):299–314.
Schindler CR, Woschek M, Franz J-N, Störmann P, Henrich D, Marzi I. Influence of Antibiotic Management on Microbial Selection and Infectious complications after Trauma. Front Med. 2021;8.
Kesinger MR, Kumar RG, Wagner AK, Puyana JC, Peitzman AP, Billiar TR, et al. Hospital-acquired pneumonia is an independent predictor of poor global outcome in severe traumatic brain injury up to 5 years after discharge. J Trauma Acute care Surg. 2015;78(2):396–402.
Li Y, Liu C, **ao W, Song T, Wang S, Incidence. Risk factors, and outcomes of Ventilator-Associated Pneumonia in Traumatic Brain Injury: a Meta-analysis. Neurocrit Care. 2020;32(1):272–85.
Dhillon NK, Adjamian N, Fierro NM, Conde G, Barmparas G, Ley EJ. Early Antibiotic Administration Is Independently Associated with Improved Survival in Traumatic Brain Injury. J Surg Res. 2022;270:495–502.
Reizine F, Asehnoune K, Roquilly A, Laviolle B, Rousseau C, Arnouat M, et al. Effects of antibiotic prophylaxis on ventilator-associated pneumonia in severe traumatic brain injury. A post hoc analysis of two trials. J Crit Care. 2019;50:221–6.
Zha S, Niu J, He Z, Fu W, Huang Q, Guan L, et al. Prophylactic antibiotics for preventing ventilator-associated pneumonia: a pairwise and bayesian network meta-analysis. Eur J Med Res. 2023;28(1):348.
Moore L, Tardif PA, Lauzier F, Bérubé M, Archambault P, Lamontagne F, et al. Low-value clinical practices in Adult Traumatic Brain Injury: an Umbrella Review. J Neurotrauma. 2020;37(24):2605–15.
Scott G, Zetterberg H, Jolly A, Cole JH, De Simoni S, Jenkins PO, et al. Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain. 2018;141(2):459–71.
Koulaeinejad N, Haddadi K, Ehteshami S, Shafizad M, Salehifar E, Emadian O, et al. Effects of Minocycline on neurological outcomes in patients with Acute Traumatic Brain Injury: a pilot study. Iran J Pharm Res. 2019;18(2):1086–96.
Mansour NO, Shama MA, Werida RH. The effect of doxycycline on neuron-specific enolase in patients with traumatic brain injury: a randomized controlled trial. Therapeutic Adv Chronic Disease. 2021;12:20406223211024362.
Zhang Q, Chen H, Zhu C, Chen F, Sun S, Liang N, et al. Efficacy and safety of intrathecal meropenem and Vancomycin in the treatment of postoperative intracranial infection in patients with severe traumatic brain injury. Exp Ther Med. 2019;17(6):4605–9.
Ganga A, Leary OP, Sastry RA, Asaad WF, Svokos KA, Oyelese AA, et al. Antibiotic prophylaxis in penetrating traumatic brain injury: analysis of a single-center series and systematic review of the literature. Acta Neurochir. 2023;165(2):303–13.
McCafferty RR, Neal CJ, Marshall SA, Pamplin JC, Rivet D, Hood BJ, et al. Neurosurgery and medical management of severe Head Injury. Mil Med. 2018;183(suppl2):67–72.
Marut D, Shammassian B, McKenzie C, Adamski J, Traeger J. Evaluation of prophylactic antibiotics in penetrating brain injuries at an academic level 1 trauma center. Clin Neurol Neurosurg. 2020;193:105777.
Petersen K, Waterman P. Prophylaxis and treatment of infections associated with penetrating traumatic injury. Expert Rev anti-infective Therapy. 2011;9(1):81–96.
Bayston R, de Louvois J, Brown EM, Johnston RA, Lees P, Pople IK. Use of antibiotics in penetrating craniocerebral injuries. Infection in Neurosurgery Working Party of British Society for Antimicrobial Chemotherapy. Lancet. 2000;355(9217):1813–7.
Hoth JJ, Franklin GA, Stassen NA, Girard SM, Rodriguez RJ, Rodriguez JL. Prophylactic antibiotics adversely affect nosocomial pneumonia in Trauma patients. J Trauma Acute Care Surg. 2003;55(2):249–54.
Maier L, Goemans CV, Wirbel J, Kuhn M, Eberl C, Pruteanu M, et al. Unravelling the collateral damage of antibiotics on gut bacteria. Nature. 2021;599(7883):120–4.
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as Major disruptors of Gut Microbiota. Front Cell Infect Microbiol. 2020;10.
Rea MC, Dobson A, O’Sullivan O, Crispie F, Fouhy F, Cotter PD, et al. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci U S A. 2011;108(1Suppl 1):4639–44.
Patangia DV, Anthony Ryan C, Dempsey E, Paul Ross R, Stanton C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen. 2022;11(1):e1260.
Mahajan C, Khurana S, Kapoor I, Sokhal S, Kumar S, Prabhakar H, et al. Characteristics of gut Microbiome after traumatic brain Injury. J Neurosurg Anesthesiol. 2023;35(1):86–90.
Hameed MQ, Hsieh TH, Morales-Quezada L, Lee HHC, Damar U, MacMullin PC et al. Ceftriaxone Treatment Preserves Cortical Inhibitory Interneuron Function via Transient Salvage of GLT-1 in a Rat Traumatic Brain Injury Model. Cerebral cortex (New York, NY: 1991). 2019;29(11):4506-18.
Cui C, Cui Y, Gao J, Sun L, Wang Y, Wang K, et al. Neuroprotective effect of ceftriaxone in a rat model of traumatic brain injury. Neurol Sci. 2014;35(5):695–700.
Wei J, Pan X, Pei Z, Wang W, Qiu W, Shi Z, et al. The beta-lactam antibiotic, ceftriaxone, provides neuroprotective potential via anti-excitotoxicity and anti-inflammation response in a rat model of traumatic brain injury. J Trauma Acute care Surg. 2012;73(3):654–60.
Goodrich GS, Kabakov AY, Hameed MQ, Dhamne SC, Rosenberg PA, Rotenberg A. Ceftriaxone treatment after traumatic brain injury restores expression of the glutamate transporter, GLT-1, reduces regional gliosis, and reduces post-traumatic seizures in the rat. J Neurotrauma. 2013;30(16):1434–41.
Garg C, Seo JH, Ramachandran J, Loh JM, Calderon F, Contreras JE. Trovafloxacin attenuates neuroinflammation and improves outcome after traumatic brain injury in mice. J Neuroinflammation. 2018;15(1):42.
Simon DW, Aneja RK, Alexander H, Bell MJ, Bayır H, Kochanek PM, et al. Minocycline attenuates high mobility Group Box 1 translocation, Microglial activation, and thalamic neurodegeneration after traumatic brain Injury in Post-natal Day 17 rats. J Neurotrauma. 2017;35(1):130–8.
Taylor AN, Tio DL, Paydar A, Sutton RL. Sex differences in thermal, stress, and inflammatory responses to Minocycline Administration in rats with traumatic brain Injury. J Neurotrauma. 2018;35(4):630–8.
Celorrio M, Shumilov K, Payne C, Vadivelu S, Friess SH. Acute minocycline administration reduces brain injury and improves long-term functional outcomes after delayed hypoxemia following traumatic brain injury. Acta Neuropathol Commun. 2022;10(1):10.
Sangobowale M, Nikulina E, Bergold PJ. Minocycline plus N-acetylcysteine protect oligodendrocytes when first dosed 12 hours after closed head injury in mice. Neurosci Lett. 2018;682:16–20.
Sangobowale MA, Grin’kina NM, Whitney K, Nikulina E, St Laurent-Ariot K, Ho JS, et al. Minocycline plus N-Acetylcysteine reduce behavioral deficits and improve histology with a clinically useful time window. J Neurotrauma. 2018;35(7):907–17.
Marjani S, Zirh S, Sever-Bahcekapili M, Cakir-Aktas C, Muftuoglu SF, Mut M. Doxycycline alleviates acute traumatic brain injury by suppressing neuroinflammation and apoptosis in a mouse model. J Neuroimmunol. 2021;359:577672.
Malek AJ, Robinson BD, Hitt AR, Shaver CN, Tharakan B, Isbell CL. Doxycycline improves traumatic brain injury outcomes in a murine survival model. J Trauma Acute care Surg. 2020;89(3):435–40.
Robinson BD, Isbell CL, Melge AR, Lomas AM, Shaji CA, Mohan CG, et al. Doxycycline prevents blood-brain barrier dysfunction and microvascular hyperpermeability after traumatic brain injury. Sci Rep. 2022;12(1):5415.
Pathare N, Sushilkumar S, Haley L, Jain S, Osier NN. The impact of traumatic Brain Injury on Microbiome Composition: a systematic review. Biol Res Nurs. 2020;22(4):495–505.
Urban RJ, Pyles RB, Stewart CJ, Ajami N, Randolph KM, Durham WJ, et al. Altered fecal Microbiome years after traumatic brain Injury. J Neurotrauma. 2020;37(8):1037–51.
Durham WJ, Foreman JP, Randolph KM, Danesi CP, Spratt H, Masel BD, et al. Hypoaminoacidemia characterizes Chronic Traumatic Brain Injury. J Neurotrauma. 2017;34(2):385–90.
George AK, Behera J, Homme RP, Tyagi N, Tyagi SC, Singh M. Rebuilding Microbiome for Mitigating Traumatic Brain Injury: Importance of Restructuring the Gut-Microbiome-Brain Axis. Mol Neurobiol. 2021;58(8):3614–27.
Celorrio M, Shumilov K, Friess SH. Gut microbial regulation of innate and adaptive immunity after traumatic brain injury. Neural Regeneration Res. 2024;19(2):272–6.
Sundman MH, Chen NK, Subbian V, Chou YH. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. 2017;66:31–44.
Patterson TT, Nicholson S, Wallace D, Hawryluk GWJ, Grandhi R. Complex feed-Forward and Feedback mechanisms Underlie the Relationship between Traumatic Brain Injury and the gut–microbiota–brain Axis. Shock. 2019;52(3).
**ong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–42.
Petersen A, Soderstrom M, Saha B, Sharma P. Animal models of traumatic brain injury: a review of pathophysiology to biomarkers and treatments. Exp Brain Res. 2021;239(10):2939–50.
Rana A, Singh S, Deshmukh R, Kumar A. Pharmacological potential of tocopherol and doxycycline against traumatic brain injury-induced cognitive/motor impairment in rats. Brain Injury. 2020;34(8):1039–50.
Cui C, Cui Y, Gao J, Sun L, Wang Y, Wang K, et al. Neuroprotective effect of ceftriaxone in a rat model of traumatic brain injury. Neurol Sciences: Official J Italian Neurol Soc Italian Soc Clin Neurophysiol. 2014;35(5):695–700.
Lim SW, Su HC, Nyam TE, Chio CC, Kuo JR, Wang CC. Ceftriaxone therapy attenuates brain trauma in rats by affecting glutamate transporters and neuroinflammation and not by its antibacterial effects. BMC Neurosci. 2021;22(1):54.
Wang S, Zhu K, Hou X, Hou L. The association of traumatic brain injury, gut microbiota and the corresponding metabolites in mice. Brain Res. 2021;1762:147450.
Opeyemi OM, Rogers MB, Firek BA, Janesko-Feldman K, Vagni V, Mullett SJ, et al. Sustained dysbiosis and decreased fecal short-chain fatty acids after traumatic Brain Injury and Impact on neurologic outcome. J Neurotrauma. 2021;38(18):2610–21.
Treangen TJ, Wagner J, Burns MP, Villapol S. Traumatic brain Injury in mice induces Acute Bacterial Dysbiosis within the fecal microbiome. Front Immunol. 2018;9:2757.
Ma EL, Smith AD, Desai N, Cheung L, Hanscom M, Stoica BA, et al. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav Immun. 2017;66:56–69.
Ritter K, Vetter D, Wernersbach I, Schwanz T, Hummel R, Schäfer MKE. Pre-traumatic antibiotic-induced microbial depletion reduces neuroinflammation in acute murine traumatic brain injury. Neuropharmacology. 2023;237:109648.
Celorrio M, Abellanas MA, Rhodes J, Goodwin V, Moritz J, Vadivelu S, et al. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol Commun. 2021;9(1):40.
Celorrio M, Shumilov K, Rodgers R, Schriefer L, Li Y, Baldridge MT, et al. Innate and peripheral Immune alterations after traumatic brain Injury are regulated in a gut microbiota-dependent manner in mice. J Neurotrauma. 2023;40(7–8):772–87.
Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158.
Kim CH. Immune regulation by microbiome metabolites. Immunology. 2018;154(2):220–9.
Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196(2):168–79.
Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26(4):515–21.
Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169(2):337–52.
Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48(6):1393–9. discussion 9-401.
Homsi S, Piaggio T, Croci N, Noble F, Plotkine M, Marchand-Leroux C, et al. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J Neurotrauma. 2010;27(5):911–21.
Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, et al. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007;204(1):220–33.
Siopi E, Calabria S, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline restores olfactory bulb volume and olfactory behavior after traumatic brain injury in mice. J Neurotrauma. 2012;29(2):354–61.
Siopi E, Cho AH, Homsi S, Croci N, Plotkine M, Marchand-Leroux C, et al. Minocycline restores sAPPα levels and reduces the late histopathological consequences of traumatic brain injury in mice. J Neurotrauma. 2011;28(10):2135–43.
Kovesdi E, Kamnaksh A, Wingo D, Ahmed F, Grunberg NE, Long JB, et al. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front Neurol. 2012;3:111.
Adembri C, Selmi V, Vitali L, Tani A, Margheri M, Loriga B et al. Minocycline but Not Tigecycline is neuroprotective and reduces the Neuroinflammatory Response Induced by the superimposition of Sepsis upon Traumatic Brain Injury*. Crit Care Med. 2014;42(8).
Siopi E, Llufriu-Dabén G, Fanucchi F, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: the effect of minocycline. Neurosci Lett. 2012;511(2):110–5.
Vonder Haar C, Anderson GD, Elmore BE, Moore LH, Wright AM, Kantor ED, et al. Comparison of the effect of minocycline and simvastatin on functional recovery and gene expression in a rat traumatic brain injury model. J Neurotrauma. 2014;31(10):961–75.
Hiskens MI, Vella RK, Schneiders AG, Fenning AS. Minocycline improves cognition and molecular measures of inflammation and neurodegeneration following repetitive mTBI. Brain Injury. 2021;35(7):831–41.
Haber M, Abdel Baki SG, Grin’kina NM, Irizarry R, Ershova A, Orsi S, et al. Minocycline plus N-acetylcysteine synergize to modulate inflammation and prevent cognitive and memory deficits in a rat model of mild traumatic brain injury. Exp Neurol. 2013;249:169–77.
Whitney K, Nikulina E, Rahman SN, Alexis A, Bergold PJ. Delayed dosing of minocycline plus N-acetylcysteine reduces neurodegeneration in distal brain regions and restores spatial memory after experimental traumatic brain injury. Exp Neurol. 2021;345:113816.
Lawless S, Bergold PJ. Better together? Treating traumatic brain injury with minocycline plus N-acetylcysteine. Neural Regeneration Res. 2022;17(12):2589–92.
Abdel Baki SG, Schwab B, Haber M, Fenton AA, Bergold PJ. Minocycline synergizes with N-Acetylcysteine and improves cognition and memory following traumatic Brain Injury in rats. PLoS ONE. 2010;5(8):e12490.
Grenier D, Huot MP, Mayrand D. Iron-chelating activity of tetracyclines and its impact on the susceptibility of Actinobacillus actinomycetemcomitans to these antibiotics. Antimicrob Agents Chemother. 2000;44(3):763–6.
Zhang L, **ao H, Yu X, Deng Y. Minocycline attenuates neurological impairment and regulates iron metabolism in a rat model of traumatic brain injury. Arch Biochem Biophys. 2020;682:108302.
Schildknecht S, Pape R, Müller N, Robotta M, Marquardt A, Bürkle A, et al. Neuroprotection by minocycline caused by direct and specific scavenging of peroxynitrite. J Biol Chem. 2011;286(7):4991–5002.
Chen-Roetling J, Chen L, Regan RF. Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun. 2009;386(2):322–6.
Zhao F, Hua Y, He Y, Keep RF, ** G. Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke. 2011;42(12):3587–93.
Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83(5):1098–116.
Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417(6884):74–8.
Gieseler A, Schultze AT, Kupsch K, Haroon MF, Wolf G, Siemen D, et al. Inhibitory modulation of the mitochondrial permeability transition by minocycline. Biochem Pharmacol. 2009;77(5):888–96.
Lopez-Rodriguez AB, Siopi E, Finn DP, Marchand-Leroux C, Garcia-Segura LM, Jafarian-Tehrani M et al. CB1 and CB2 cannabinoid receptor antagonists prevent minocycline-induced neuroprotection following traumatic brain injury in mice. Cerebral cortex (New York, NY: 1991). 2015;25(1):35–45.
Bergold PJ, Furhang R, Lawless S. Treating traumatic Brain Injury with Minocycline. Neurotherapeutics. 2023;20(6):1546–64.
Pechacek KM, Reck AM, Frankot MA, Vonder Haar C. Minocycline fails to treat chronic traumatic brain injury-induced impulsivity and attention deficits. Exp Neurol. 2022;348:113924.
Hanlon LA, Huh JW, Raghupathi R. Minocycline transiently reduces Microglia/Macrophage activation but exacerbates cognitive deficits following repetitive traumatic brain Injury in the neonatal rat. J Neuropathol Exp Neurol. 2016;75(3):214–26.
Cheng S, Hou J, Zhang C, Xu C, Wang L, Zou X, et al. Minocycline reduces neuroinflammation but does not ameliorate neuron loss in a mouse model of neurodegeneration. Sci Rep. 2015;5(1):10535.
Kelso ML, Scheff NN, Scheff SW, Pauly JR. Melatonin and minocycline for combinatorial therapy to improve functional and histopathological deficits following traumatic brain injury. Neurosci Lett. 2011;488(1):60–4.
Wood H. Traumatic brain injury: Minocycline reduces microglial activation but increases neurodegeneration after TBI. Nat Reviews Neurol. 2018;14(3):127.
Meythaler J, Fath J, Fuerst D, Zokary H, Freese K, Martin HB, et al. Safety and feasibility of minocycline in treatment of acute traumatic brain injury. Brain Injury. 2019;33(5):679–89.
Nagamoto-Combs K, McNeal DW, Morecraft RJ, Combs CK. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma. 2007;24(11):1719–42.
Nagamoto-Combs K, Morecraft RJ, Darling WG, Combs CK. Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury. J Neurotrauma. 2010;27(3):565–85.
Safaiyan S, Besson-Girard S, Kaya T, Cantuti-Castelvetri L, Liu L, Ji H, et al. White matter aging drives microglial diversity. Neuron. 2021;109(7):1100–17e10.
Strickland BA, Bakhsheshian J, Emmanuel B, Amar A, Giannotta SL, Russin JJ, et al. Neuroprotective effect of minocycline against acute brain injury in clinical practice: a systematic review. J Clin Neuroscience: Official J Neurosurgical Soc Australasia. 2021;86:50–7.
Ghiam MK, Patel SD, Hoffer A, Selman WR, Hoffer BJ, Hoffer ME. Drug Repurposing in the treatment of traumatic brain Injury. Front NeuroSci. 2021;15:635483.
Daglas M, Adlard PA. The involvement of Iron in Traumatic Brain Injury and neurodegenerative disease. Front NeuroSci. 2018;12:981.
Juan SMA, Daglas M, Truong PH, Mawal C, Adlard PA. Alterations in iron content, iron-regulatory proteins and behaviour without tau pathology at one year following repetitive mild traumatic brain injury. Acta Neuropathol Commun. 2023;11(1):118.
Tourville A, Viguier S, González-Lizárraga F, Tomas-Grau RH, Ramirez P, Brunel JM et al. Rescue of dopamine neurons from Iron-dependent ferroptosis by doxycycline and demeclocycline and their non-antibiotic derivatives. Antioxid (Basel). 2023;12(3).
Faruk M, Daud KR, Islam AA, Ihwan A, Alfian Zainuddin AA. Oral doxycycline on the level of the matrix metalloproteinase 9 in rat models of experiencing traumatic brain injury. IIUM Med J Malaysia. 2021;20(1)
Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA, Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65(4):702–8.
Minta K, Brinkmalm G, Al Nimer F, Thelin EP, Piehl F, Tullberg M, et al. Dynamics of cerebrospinal fluid levels of matrix metalloproteinases in human traumatic brain injury. Sci Rep. 2020;10(1):18075.
Burmeister M, Fraunenstein A, Kahms M, Arends L, Gerwien H, Deshpande T, et al. Secretomics reveals gelatinase substrates at the blood-brain barrier that are implicated in astroglial barrier function. Sci Adv. 2023;9(29):eadg0686.
Koistinaho M, Malm TM, Kettunen MI, Goldsteins G, Starckx S, Kauppinen RA, et al. Minocycline protects against permanent cerebral ischemia in wild type but not in matrix metalloprotease-9-deficient mice. J Cereb Blood Flow Metab. 2005;25(4):460–7.
Feng D, Wang W, Dong Y, Wu L, Huang J, Ma Y, et al. Ceftriaxone alleviates early brain injury after subarachnoid hemorrhage by increasing excitatory amino acid transporter 2 expression via the PI3K/Akt/NF-κB signaling pathway. Neuroscience. 2014;268:21–32.
Romariz SAA, Main BS, Harvey AC, Longo BM, Burns MP. Delayed treatment with ceftriaxone reverses the enhanced sensitivity of TBI mice to chemically-induced seizures. PLoS ONE. 2023;18(7):e0288363.
Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem. 2008;283(19):13116–23.
Rao GN, Jupudi S, Pant P, Palathoti N, Rajagopal K, Govindasamy R, et al. Ceftriaxone induces glial EAAT-2 promotor region via NF-kB conformational changes: an interaction analysis using HADDOCK. J Cell Biochem. 2023;124(3):359–72.
Obrenovitch TP, Urenjak J. Is high Extracellular Glutamate the Key to Excitotoxicity in Traumatic Brain Injury? J Neurotrauma. 1997;14(10):677–98.
Andrew RD, Farkas E, Hartings JA, Brennan KC, Herreras O, Müller M, et al. Questioning glutamate excitotoxicity in Acute Brain damage: the importance of spreading depolarization. Neurocrit Care. 2022;37(1):11–30.
Tehse J, Taghibiglou C. The overlooked aspect of excitotoxicity: glutamate-independent excitotoxicity in traumatic brain injuries. Eur J Neurosci. 2019;49(9):1157–70.
Wilkie CM, Barron JC, Brymer KJ, Barnes JR, Nafar F, Parsons MP. The effect of GLT-1 Upregulation on Extracellular Glutamate dynamics. Front Cell Neurosci. 2021;15:661412.
Schmuck G, Schürmann A, Schlüter G. Determination of the excitatory potencies of fluoroquinolones in the Central Nervous System by an in vitro model. Antimicrob Agents Chemother. 1998;42(7):1831–6.
Lode H. Potential interactions of the extended-spectrum fluoroquinolones with the CNS. Drug Saf. 1999;21(2):123–35.
Poon IKH, Chiu Y-H, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, et al. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature. 2014;507(7492):329–34.
Rusiecka OM, Tournier M, Molica F, Kwak BR. Pannexin1 channels-a potential therapeutic target in inflammation. Front Cell Dev Biol. 2022;10:1020826.
Makarenkova HP, Shestopalov VI. The role of pannexin hemichannels in inflammation and regeneration. Front Physiol. 2014;5:63.
Adamson SE, Leitinger N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 2014;588(8):1416–22.
Seo JH, Dalal MS, Contreras JE. Pannexin-1 channels as mediators of Neuroinflammation. Int J Mol Sci. 2021;22(10).
Gölz C, Kirchhoff FP, Westerhorstmann J, Schmidt M, Hirnet T, Rune GM, et al. Sex hormones modulate pathogenic processes in experimental traumatic brain injury. J Neurochem. 2019;150(2):173–87.
Seo JH, Dalal MS, Calderon F, Contreras JE. Myeloid Pannexin-1 mediates acute leukocyte infiltration and leads to worse outcomes after brain trauma. J Neuroinflammation. 2020;17(1):245.
Lee KE, Cho KO, Choi YS, Kim SY. The neuroprotective mechanism of ampicillin in a mouse model of transient forebrain ischemia. Korean J Physiol Pharmacol. 2016;20(2):185–92.
Simon DW, Rogers MB, Gao Y, Vincent G, Firek BA, Janesko-Feldman K, et al. Depletion of gut microbiota is associated with improved neurologic outcome following traumatic brain injury. Brain Res. 2020;1747:147056.
Lai JCY, Svedin P, Ek CJ, Mottahedin A, Wang X, Levy O et al. Vancomycin is protective in a neonatal mouse model of Staphylococcus epidermidis-potentiated hypoxic-ischemic brain Injury. Antimicrob Agents Chemother. 2020;64(3).
Chen B, Cao H, Chen L, Yang X, Tian X, Li R, et al. Rifampicin attenuated Global Cerebral Ischemia Injury via activating the Nuclear factor erythroid 2-Related factor pathway. Front Cell Neurosci. 2016;10:273.
Bi W, Zhu L, Wang C, Liang Y, Liu J, Shi Q, et al. Rifampicin inhibits microglial inflammation and improves neuron survival against inflammation. Brain Res. 2011;1395:12–20.
Tomiyama T, Shoji A, Kataoka K, Suwa Y, Asano S, Kaneko H, et al. Inhibition of amyloid beta protein aggregation and neurotoxicity by rifampicin. Its possible function as a hydroxyl radical scavenger. J Biol Chem. 1996;271(12):6839–44.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
K.R., E.V.G., and M.K.E.S. conducted literature research and wrote the manuscript. P.S and L.H. conducted literature research and created the tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ritter, K., Somnuke, P., Hu, L. et al. Current state of neuroprotective therapy using antibiotics in human traumatic brain injury and animal models. BMC Neurosci 25, 10 (2024). https://doi.org/10.1186/s12868-024-00851-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-024-00851-6