Abstract
Background
Green-fleshed radish (Raphanus sativus L.) is an economically important root vegetable of the Brassicaceae family, and chlorophyll accumulates in its root tissues. It was reported that the basic helix-loop-helix (bHLH) transcription factors play vital roles in the process of chlorophyll metabolism. Nevertheless, a comprehensive study on the bHLH gene family has not been performed in Raphanus sativus L.
Results
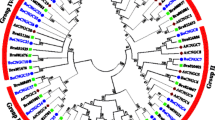
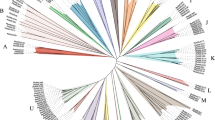
In this study, a total of 213 Raphanus sativus L. bHLH (RsbHLH) genes were screened in the radish genome, which were grouped into 22 subfamilies. 204 RsbHLH genes were unevenly distributed on nine chromosomes, and nine RsbHLH genes were located on the scaffolds. Gene structure analysis showed that 25 RsbHLH genes were intron-less. Collineation analysis revealed the syntenic orthologous bHLH gene pairs between radish and Arabidopsis thaliana/Brassica rapa/Brassica oleracea. 162 RsbHLH genes were duplicated and retained from the whole genome duplication event, indicating that the whole genome duplication contributed to the expansion of the RsbHLH gene family. RNA-seq results revealed that RsbHLH genes had a variety of expression patterns at five development stages of green-fleshed radish and white-fleshed radish. In addition, the weighted gene co-expression network analysis confirmed four RsbHLH genes closely related to chlorophyll content.
Conclusions
A total of 213 RsbHLH genes were identified, and we systematically analyzed their gene structure, evolutionary and collineation relationships, conserved motifs, gene duplication, cis-regulatory elements and expression patterns. Finally, four bHLH genes closely involved in chlorophyll content were identified, which may be associated with the photosynthesis of the green-fleshed radish. The current study would provide valuable information for further functional exploration of RsbHLH genes, and facilitate clarifying the molecular mechanism underlying photosynthesis process in green-fleshed radish.
Similar content being viewed by others
Background
Radish (Raphanus sativus L.), belonging to the Brassicaceae family, is an important root vegetable crop with multiple varieties, such as green-fleshed radish. Its flesh is green due to the existence of chlorophyll, which is necessary for photosynthesis. The expression of chlorophyll biosynthesis-related genes contributes to the chlorophyll accumulation. Due to the presence of chlorophyll, chlorophyll fluorescence technology has detected the occurrence of photosynthesis in the flesh of green-fleshed radish [1].
Basic Helix-Loop-Helix (bHLH) transcription factor family is the second largest gene families in plant kingdom, usually classified into 15–26 subfamilies. Members of the bHLH gene family have been identified in many species, for example, 188 in apple (divided into 18 subfamilies, [2]), 159 in tomato (divided into 25 subfamilies, [3]) and 115 in grape (classified into 25 subfamilies, [4]). BHLH transcription factors have a highly conserved bHLH domain with ∼60 amino acids, which comprises a basic region followed by two amphipathic α-helices separated by a variable loop region (HLH) [5]. The basic region is relevant to the DNA binding that allows the bHLH proteins binding to the cis-acting elements in the promoter regions of the target genes, and the HLH region functions as a dimerization domain that allows the formation of homo- and/or heterodimers.
The researches show that the bHLH proteins can positively or negatively regulate the process of chlorophyll biosynthesis. Phytochrome interacting factor1(PIF1), as a bHLH protein, negatively controls the chlorophyll biosynthesis in the dark by regulating the expression of genes coding heme oxygenase (HO3), protochlorophyllide oxidoreductase (POR), and ferrochelatase (FeChII), which are involved in the chlorophyll biosynthetic pathway [6]. Ectopic overexpression of a bHLH gene from Populus euphratica enhances tolerance to water-deficit stress and results in a higher chlorophyll content and photosynthetic rate in Arabidopsis [12]. BHLH genes have been confirmed in B. rapa and B. oleracea, with 251 B. rapa bHLHs and 268 B. oleracea bHLHs, respectively [13]. To understand the synteny relationships between RsbHLH genes and Bra/BolbHLH genes, the syntenic orthologous gene pairs were identified between RsbHLHs and Bra/BolbHLHs. There were 950 syntenic orthologous gene pairs between 174 RsbHLHs and 209 BrabHLHs (Fig. 6b). A total of 20 RsbHLHs only one syntenic orthologous BrabHLHs, such as RsbHLH6, RsbHLH19, RsbHLH36. The remaining RsbHLHs had at least two syntenic orthologous BrabHLHs. RsbHLHs with two syntenic BrabHLHs are the most, such as RsbHLH61- BrabHLH080/BrabHLH218. The number of RsbHLHs with four syntenic orthologous BrabHLHs ranked the second, such as RsbHLH52-BrabHLH052/BrabHLH168/BrabHLH038/BrabHLH006. Of course, there was also a case where multiple RsbHLHs correspond to one syntenic orthologous BrabHLH, such as RsbHLH14/RsbHLH98/RsbHLH110/RsbHLH149-BrabHLH081. There were 638 syntenic orthologous gene pairs between 162 RsbHLHs and 187 BolbHLHs (Fig. 6b). Like BrabHLHs, the syntenic relationship between RsbHLHs and BolbHLHs was divided into 1:1, 1:n, and n:1(n ≥ 2). A total of 24 RsbHLHs only one syntenic orthologous BolbHLHs, such as RsbHLH37, RsbHLH121, and RsbHLH128. RsbHLHs with two syntenic orthologous BolbHLHs had the highest number (such as RsbHLH61-BolbHLH156/BolbHLH137), followed by RsbHLHs with three syntenic orthologous BolbHLHs (RsbHLH34-BolbHLH070/BolbHLH157/BolbHLH213). Likewise, there were also multiple RsbHLHs corresponding to one syntenic orthologous BolbHLH, such as RsbHLH8/RsbHLH40/RsbHLH175/RsbHLH193- BolbHLH080. To sum up, although the number of bHLH genes in B. oleracea was slightly more than that of B. rapa, the syntenic orthologous bHLH pairs between B. oleracea and R. sativus was significantly less than that of B. rapa and R. sativus, which may be due to the closer relationship between B. rapa and R. sativus. This is supported by the genetic studies that the size and structural characteristics of the R. sativus genome are similar to those of B. rapa genome [14].
Genome duplication events can contribute to the expansion of gene family in plant kingdom. The results suggested that four types of duplication existed in the RsbHLH members, namely whole genome duplication (WGD) or segmental event, dispersed event, proximal event, and tandem event (Additional file 3). 162 (76%) RsbHLH genes were duplicated and retained from a WGD or segmental event, revealing that WGD or segmental duplication was the main driving force for the expansion of the radish bHLH gene family. Eight tandem events of 16 RsbHLH genes were identified and located on four chromosomes. Among these events, two events (RsbHLH10 and RsbHLH11, RsbHLH96 and RsbHLH97) were located on chromosome R1 and R5, respectively. Three events (RsbHLH57 and RsbHLH58, RsbHLH59 and RsbHLH60, and RsbHLH69 and RsbHLH70) took place within the same chromosome R4, and the remaining three events (RsbHLH133 and RsbHLH134, RsbHLH136 and RsbHLH137, and RsbHLH144 and RsbHLH145) also took place within the same chromosome R6. Finally, the dN / dS for the 48 paralogous gene pairs were calculated to confirm the selection pressure (Additional file 4). All of the RsbHLH paralogous gene pairs had a dN / dS < 1(dN means non-synonymous substitution ratio; dS means synonymous substitution ratio), implying that these RsbHLH genes had experienced strong purifying selective pressure.
Promoter cis-element analysis
BHLH genes can take part in the regulation of plant growth and development, and response to various abiotic stresses. To further investigate the potential biological functions of RsbHLH genes, the cis-acting regulatory elements in the promoter regions of RsbHLH genes were analyzed using PLACE tool. As shown in Fig. 7 and Additional file 5, three main categories were identified in the cis-acting regulatory elements of RsbHLH genes. Category one was associated with plant growth and development, such as flavonoid biosynthesis, phytochrome expression, and circadian control. The motifs contained in this category were MBSI, circadian, CAT-box, RY-element, etc. Light responsive element was present in the promoter regions of 208 RsbHLH genes, indicating that the expression of RsbHLH genes maybe controlled by light. The genes involved in chlorophyll metabolism are regulated by light [15]. This may mean that RsbHLH genes had a certain relationship with chlorophyll metabolism. Category two was related to phytohormones, such as gibberellin, methyl jasmonate, and abscisic acid. The motifs included in this category were CGTCA-motif, ABRE, TCA-element, P-box, etc. Category three was involved in abiotic stresses, such as low-temperature responsiveness, light responsiveness, and drought-inducibility. The motifs included in this category were LTR, G-box, MBS, WUN-motif, etc.
Analysis of GO enrichment and expression
To predict the potential biological functions, the GO enrichment analysis was performed by WEGO to show three aspects of functional classifications, namely, cellular component, molecular function, and biological process (Fig. 8). Among the 213 RsbHLH genes, 207 RsbHLH genes were enriched in the biological process, and most of these RsbHLHs mainly participated in “metabolic process” (such as “primary metabolic process” and “biosynthetic process”), and “biological regulation” (such as “regulation of metabolic process” and “regulation of cellular process”). Additionally, 85 RsbHLHs, 74 RsbHLHs and 72 RsbHLHs were involved in “response to stimulus”, “developmental process”, and “multicellular organismal process”, respectively. The “response to stimulus” was mainly associated with “response to abiotic stimulus” (cold, salt, light, etc.) and “response to chemical” (gibberellin, abscisic acid, jasmonic acid, etc.), which was consistent with the previous promoter element analysis. The “developmental process” involved mainly in “anatomical structure development” (fruit development, flower development, carpel development, etc.). The “multicellular organismal process” contained mainly photomorphogenesis, guard cell differentiation, root hair initiation and so on. In summary, RsbHLHs could play an important role in the growth and development of radish.
A comparative RNA-seq analysis between the green-fleshed radish (GF) and white-fleshed radish (WF) was made to study the expression patterns of 213 RsbHLHs at five growth stages. The RsbHLH genes with FPKM < 1 at all five stages in GF and WF were regarded as unexpressed genes, so only 119 RsbHLH genes were carried on expression analysis. The expression patterns of these 119RsbHLHs among five stages varied greatly (Fig. 9). For example, whether GF or WF, some RsbHLHs were stably expressed at five stages, such as RsbHLH29. Additionally, whether GF or WF, RsbHLH105 and RsbHLH154 were only highly expressed at the stage 3. For RsbHLH36, it had always been highly expressed at five stages in GF, while always lowly expressed at five stages in WF. Reversely, RsbHLH140 had always been highly expressed at five stages in WF, while always lowly expressed at five stages in GF. For RsbHLH130, it only had a relatively high expression at the stage 3 of WF. The various expression patterns of RsbHLHs among five stages suggested that the bHLH members may play a vital role in the entire growth and development of radish taproot. The differentially expressed gene (DEG) analysis revealed that there were 19, 16, 22, 18, 13 differentially expressed RsbHLHs between GF and WF at stage 1 to stage 5, respectively (Additional file 6). Four RsbHLHs (RsbHLH36, RsbHLH44, RsbHLH69, and RsbHLH140) were differentially expressed genes shared by the five stages. The differential expression of RsbHLHs may lead to the differences in traits between green-fleshed radish and white-fleshed radish.
To demonstrate the accuracy and reproducibility of the transcriptome data, fifteen RsbHLH genes were selected to analyze the transcript abundance by qRT-PCR. The results showed that the expression trends of these fifteen genes detected by qRT-PCR in the fifth stage were in line with the RNA-Seq results (Additional files 7 and 8). Therefore, the reliability of the transcriptome data was confirmed.
Candidate bHLHs involved in chlorophyll metabolism in Raphanus sativus L
The weighted gene co-expression network analysis (WGCNA) was used to analyze the connection between genes and physiological traits, discovering the vital genes associated with physiological traits. There were 8666 DEGs between GF and WF, including 46 bHLH genes, and these DEGs were performed by WGCNA. The expression profiles of the 8666 genes were grouped into 16 modules (MEs), displaying 15 different co-expression networks (ME1-ME15) and the outliers that did not belong to any cluster (ME0). As Fig. 10a shown, different colors represented different modules, with the module size ranging from 27 to 2510. To confirm modules that were significantly associated with chlorophyll content, the module-trait correlation relationships were constructed (Fig. 10b). Chlorophyll a content was significantly negatively correlated with the turquoise module (-0.9) and midnightblue module (-0.7), and significantly positively associated with the blue module (0.86) and brown module (0.72). Chlorophyll b was significantly positively correlated with the blue module (0.8), and significantly negatively correlated with the turquoise module (-0.8). These four modules contained 23 bHLH genes, which were used to screen out hub genes.
a Hierarchical cluster tree revealing gene co-expression modules identified by WGCNA. The branches contain 15 modules labeled in different colors. Except the gray module, the modules are named ME1 to ME15. b Module-trait associations. Columns correspond to chlorophyll content, and rows correspond to the characteristic genes of the modules. The correlation between two is shown in cell by Pearson correlation coefficient, and p-value is in parentheses. Cell color ranges from red (high positive correlation) to blue (high negative correlation)
Gene with high within-module connectivity was considered as a hub gene in a module [16]. Hub genes in modules could be more important than the other genes in the co-expression network, and they were considered as the representatives of the modules. Based on the high connectivity, four bHLH genes were regarded as hub genes: RsbHLH140 (turquoise), RsbHLH52 (blue), and RsbHLH36 and RsbHLH49 (brown). DEGs co-expressed with these four bHLH genes were screened out for further analysis.
Two DEG genes (Rs498020 and Rs428920) co-expressed with RsbHLH52 and three DEG genes (Rs536440, Rs386330, and Rs340620) co-expressed with RsbHLH140 were found to be involved in the chlorophyll metabolic pathway [1], and these five genes were shown in Fig. 11. Furthermore, as shown in Fig. 10b, RsbHLH140 was negatively correlated with chlorophyll content, while RsbHLH36, RsbHLH49, and RsbHLH52 were positively correlated with chlorophyll content. Thus, it was concluded that RsbHLH140 could negatively regulated the process of chlorophyll metabolism, and RsbHLH36, RsbHLH49, and RsbHLH52 positively controlled the process of chlorophyll metabolism, which was consistent with the analysis results of the gene expression pattern (Fig. 9). To sum up, these four bHLH genes may be involved in the metabolic process of chlorophyll, and then associated with GF photosynthesis.
Discussion
The whole genome duplication greatly facilitates the expansion of the bHLH gene family
The whole genome duplication (WGD) events have occurred throughout the process of plant evolution, which was a driving force for the expansion of gene family [17]. In radish, some gene families expanded also mainly through the whole genome duplication to generate the large number of members, such as MYB gene family, HSF gene family and CPA gene family [18,19,20]. In this study, 213 bHLH genes were identified from the radish genome, showing that the bHLH gene family in radish had been expanded in comparison with that in Arabidopsis. The results showed that 162 (76%) RsbHLH genes were duplicated and retained in the WGD event, implying that WGD event greatly promoted the amplification of the RsbHLH gene family. This result was consistent with the results of the bHLH gene family analysis in other species, such as Nelumbo nucifera, Fagopyrum tataricum, and Xanthoceras sorbifolia Bunge [21,22,23].
Gene duplication promotes the formation of the paralogous gene pairs. A total of 48 paralogous gene pairs were identified in our results, including 8 paralogous gene pairs from the tandem duplication and 36 paralogous gene pairs from the WGD duplication. Among these paralogous gene pairs, 16 paralogous gene pairs had the different gene structure, and exon gain/loss was observed. For instance, RsbHLH17 contained four exons, while its paralogous RsbHLH170 had seven exons (Fig. 2), indicating a gain of three exons occurred during evolution. RsbHLH138 contained six exons, while its paralogous RsbHLH193 had four exons, indicating a loss of two exons occurred during evolution. A similar pattern was also reported in bHLH gene families of the Ginkgo biloba and Xanthoceras sorbifolia Bunge [23, 24]. These gain/losses could be due to the results of chromosomal rearrangements and fusions, and may potentially give rise to the functional diversification of the gene families [25]. Additionally, 12 paralogous gene pairs had the discrepant motifs, in which four gene pairs had the same gene structures, such as RsbHLH10-RsbHLH11 and RsbHLH74-RsbHLH152. Differences in gene sequences may lead to changes in protein domains although they had the same gene structures. These proteins may have gone through the wide domain shuffling during the WGD [26]. We also found that despite some paralogous gene pairs had the different gene structures, they had the same motifs, such as RsbHLH52-RsbHLH161 and RsbHLH93-RsbHLH124, revealing that the sequences of protein motifs were conserved in the process of evolution.
The duplicated genes could acquire the new functions or segment the original functions to improve the adaptability of environments [27]. There are four fates for duplicated genes: (1) duplicated genes retain original functions; (2) a copy of the duplicated genes is silenced; (3) a copy of the duplicated genes retains the original function, while another copy obtains the new function, called neofunctionalization; (4) Two copies segment the original functions and obtain different functions, called subfunctionalization [28, 29]. The functional divergence of duplicated genes could cause the alteration in the expression pattern. Herein, some paralogous gene pairs showed the different expression patterns. For example, RsbHLH2 was expressed at five stages of the GF and WF, while its paralogous RsbHLH100 was not expressed at five stages of the GF and WF. RsbHLH92 had the lowest expression level at the third stage of the GF and WF, while its paralogous RsbHLH123 had the highest expression level at the third stage of the GF and WF. In a word, the distinct expression patterns of the paralogous bHLH gene pairs may lead to the formation of the unique traits and improving the adaptability to the environment in radish.
BHLH genes may have important functions in the photosynthesis in green-fleshed radish
Transcription factors (TFs) are activated and bind to the promoter of the crucial genes involved in various biological pathways, and regulate the growth and development of plants. As one of the most important biological pathways for plants, photosynthesis is regulated by a variety of transcription factors [30,31,32]. The bHLH gene family is the second largest TF family, playing an important role in the regulation of photosynthesis in plants [33].
In addition to leaves, photosynthesis also appears in many non-foliar organs. The results of chlorophyll fluorescence show that photosynthesis can occur in the green-fleshed radish rich in chlorophyll [1]. In our study, four bHLH genes (RsbHLH36, RsbHLH49, RsbHLH52, and RsbHLH140) may participate in controlling the photosynthesis process by influencing the changes of chlorophyll content. In the bHLH gene family, a few members are generally considered as negative regulators of photosynthesis. For example, phytochrome interacting factor1 (PIF1) and phytochrome interacting factor4 (PIF4), they negatively regulate the chlorophyll biosynthesis or bring about the chlorophyll degradation to lower the chlorophyll content, hindering the progress of photosynthesis [6, 9]. According to the WGCNA analysis, RsbHLH140 was significantly negatively correlated with the chlorophyll content. In addition, compared with GF, RsbHLH140 had the higher expression level in WF (Fig. 9). Three DEGs (Rs536440, Rs386330, and Rs340620) co-expressed with RsbHLH140 were involved in chlorophyll biosynthesis pathway, and they were expressed in GF, while hardly expressed in WF [1], showing that the higher expression of RsbHLH140 may suppress the expression of these three genes in WF. The A. thaliana orthologous genes of these three genes (AT3G56940, AT1G74470, and AT3G51820) are annotated in the TAIR database and participate in the chlorophyll biosynthetic process [34]. So, RsbHLH140 may act as a negative regulator of photosynthesis by suppress the chlorophyll biosynthesis. It is reported that knocking out of the negatively regulated bHLH gene can enhance the photosynthesis. Chen et al. [35] find that knocking out NRP1 gene, as a bHLH gene, gives rise to greater photosynthesis and increased biomass in rice.
Additionally, a few bHLH genes are regarded as positive regulators of photosynthesis. In contrast to PIF1, PIF3 has been proved as a positive regulator of photosynthesis. PIF3 contributes to the chlorophyll accumulation and acts positively in chloroplast development [36]. Overexpression of PebHLH35 from Populus euphratica results in a higher chlorophyll content and enhance the photosynthetic rate [Search of cis-elements in the RsbHLH gene promoter regions The upstream 2000 bp genomic sequences of RsbHLH genes relative to the translation start codon were extracted from Raphanus sativus L. genome. These 2000 bp regions were regarded as the promoter sequences of RsbHLH genes. The cis-regulatory elements of RsbHLH genes were screened from these promoter regions using online tool PLACE (https://www.dna.affrc.go.jp/PLACE/?action=newplace). Total RNA was extracted from the flesh tissues using Trizol reagent (Promega, Madison, WI, USA). The purity, concentration, and integrity of RNA were detected by the NanoPhotometer® spectrophotometer (IMPLEN, CA, USA), the Qubit® 2.0 Flurometer (Life Technologies, CA, USA), and the Bioanalyzer 2100 system (Agilent Technologies, CA, USA), respectively. After the RNA quality assessment, 30 sequencing libraries were generated using the NEBNext® Ultra™ Directional RNA Library Prep Kit according to the Illumina manufacturer's protocols (NEB, USA). The quality of libraries was checked by the Agilent Bioanalyzer 2100 system. Finally, 150 bp paired-end sequencing was performed on an Illumina Hiseqxten platform. After screening, the high-quality clean reads were aligned to the reference Raphanus sativus L. genome using the HISAT2 software [41]. The mapped clean reads were calculated to obtain the read count for each gene according to the map** results by the featureCounts software [42]. The expression level of each gene was estimated using the fragments per kilobase of exon per million mapped reads (FPKM) value, which was calculated using the countToFPKM package (https://github.com/AAlhendi1707/countToFPKM). The formula is as follows: \(\text{FPKM} = \frac{{10}^{6}{\text{C}}}{\text{NL/}{10}^{3}}\), where C is the number of fragments that specially mapped to the gene, N is the total number of fragments that specially mapped to the reference genome, and L is the number of bases in the coding region of the gene. Genes were considered to be differentially expressed genes (DEGs) between GF and WF with FDR value ≤ 0.05, Padj value < 0.01and |log2 FC|≥ 1.5 based on the DEGSeq R package [43]. The expression patterns of RsbHLH genes in the five developmental stages were presented by the TBtools software [44]. Fifteen bHLH genes were randomly chosen to be verified by qRT-PCR. The specific primers of fifteen genes were designed by the Primer5 software, and the primer sequences were listed in Additional file 9. QRT-PCR was performed using the Bio-Rad Real-Time PCR platform with quant one step qRT-PCR Kit (Tian gen). Actin gene was used as the internal control to standardize the results, and 2−ΔΔCT method was applied to calculate the relative expression level [45]. The all reactions were carried out with the following conditions: 95 °C for 15 min and 40 cycles of 95 °C for 10 s, 60 °C for 30 s. After each run, a melting curve was generated to ensure the product specificity and to check for the presence of primer dimers. The co-expression network of DEGs was constructed using WGCNA package in Rstudio [46]. The PickSoftThreshhold function was used to confirm a soft threshold (power) value according to the approximate Scale-free Topology Criterion. The soft threshold was 10 to establish the co-expression network on the basis of the adjacency matrix. The automatic network construction function blockwiseModules was applied to obtain weighted co-expression clusters, called modules, with the following operating parameters: power = 10, TOMType = unsigned, minModuleSize = 30, reassignThreshold = 0, minKMEtoStay = 0.3, mergeCutHeight = 0.25. The data on chlorophyll content was derived from our previous research results [1].RNA-seq and qRT-PCR analysis of RsbHLH genes
The weighted gene co-expression network analysis
Availability of data and materials
The raw data of RNA-seq had been deposited to Sequence Read Archive (SRA) in NCBI (accession number: PRJNA684971).
Abbreviations
- BHLH:
-
Basic helix-loop-helix
- GSDS:
-
Gene Structure Display Server
- MEME:
-
Multiple Em for Motif Elicitation
- HMM:
-
Hidden Markov Model
- dS:
-
Synonymous
- dN:
-
Non-synonymous
- NJ:
-
Neighbor-Joining
- FPKM:
-
Fragments per kilobase of exon per million mapped reads
- WGD:
-
Whole genome duplication
- DEG:
-
Differentially expressed gene
- qRT-PCR:
-
Quantitative Real-Time Polymerase Chain Reaction
- WGCNA:
-
Weighted gene co-expression network analysis
References
Li YY, Han M, Wang RH, Gao MG. Comparative transcriptome analysis identifies genes associated with chloro-phyll levels and reveals photosynthesis in green flesh of radish taproot. PLoS ONE. 2021;16:5.
Mao K, Dong QL, Li C, Liu CH, Ma FW. Genome wide identification and characterization of apple bHLH tran-scription factors and expression analysis in response to drought and salt stress. Front Plant Sci. 2017;8:480.
Sun H, Fan HJ, Ling HQ. Genome-wide identification and characterization of the bHLH gene family in tomato. BMC Genomics. 2015;16:9.
Li M, Sun L, Gu H, Cheng DW, Guo XZ, Chen R, et al. Genome-wide characterization and analysis of bHLH transcription factors related to anthocyanin biosynthesis in spine grapes (Vitis davidii). Sci Rep. 2021;11:6863.
Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15:1749–70.
Moon J, Zhu L, Shen H, Huq E. PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in Arabidopsis. P Natl Acad Sci USA. 2008;105:9433–8.
Dong Y, Wang CP, Han X, Tang S, Liu S, **a XL, et al. A novel bHLH transcription factor PebHLH35 from Populus euphratica confers drought tolerance through regulating stomatal development, photosynthesis and growth in Arabidopsis. Biochem Bioph Res Co. 2014;450:453–8.
Dong HZ, Chen QM, Dai YQ, Hu WJ, Zhang SL, Huang XS. Genome-wide identification of PbrbHLH family genes, and expression analysis in response to drought and cold stresses in pear (Pyrus bretschneideri). BMC Plant Biol. 2021;21:86.
Song Y, Yang CW, Gao S, Zhang W, Li L, Kuai BK. Age-triggered and dark-induced leaf senescence require the bHLH Transcription Factors PIF3, 4, and 5. Mol Plant. 2014;7:1776–87.
Cheng F, Wu J, Fang L, Wang XW. Syntenic gene analysis between Brassica rapa and other Brassicaceae species. Front Plant Sci. 2012;3:198.
Inaba R, Nishio T. Phylogenetic analysis of Brassiceae based on the nucleotide sequences of the S-locus related gene, SLR1. Theor Appl Genet. 2002;105:1159–65.
Mun JH, Chung H, Chung WH, Oh M, Jeong YM, Kim N, et al. Construction of a reference genetic map of Raphanus sativus based on genoty** by whole-genome resequencing. Theor Appl Genet. 2015;128:259–72.
Miao LM, Gao YY, Zhao K, Kong LJ, Yu SB, Li RR, et al. Comparative analysis of basic helix–loop–helix gene family among Brassica oleracea, Brassica rapa, and Brassica napus. BMC Genomics. 2020;21:1–18.
Jeong YM, Kim N, Ahn BO, Oh M, Chung WH, Chung H, et al. Elucidating the triplicated ancestral genome structure of radish based on chromosome-level comparison with the Brassica genomes. Theor Appl Genet. 2016;129:1357–72.
Masuda T, Fujita Y. Regulation and evolution of chlorophyll metabolism. Photoch Photobio Sci. 2008;7:1131–49.
Tang JN, Kong DG, Cui QX, Wang K, Zhang D, Gong Y, et al. Prognostic genes of breast cancer identified by gene co-expression network analysis. Front Oncol. 2018;8:374.
Rensing SA. Gene duplication as a driver of plant morphogenetic evolution. Curr Opin Plant Biol. 2014;17:43–8.
Tang MJ, Xu L, Wang Y, Cheng WW, Luo XB, **e Y, et al. Genome-wide characterization and evolutionary analysis of heat shock transcription factors (HSFs) to reveal their potential role under abiotic stresses in radish (Raphanus sativus L.). MC Genomics. 2019;20:772.
Wang Y, Ying JL, Zhang Y, Xu L, Zhang WT, Ni M, et al. Genome-wide identification and functional characterization of the cation proton antiporter (CPA) family related to salt stress response in radish (Raphanus sativus L.). Int J Mol Sci. 2020;21:8262.
Muleke EM, Wang Y, Zhang WT, Xu L, Ying JL, Karanja BK, et al. enome-wide identification and expression profiling of MYB transcription factor genes in radish (Raphanus sativus L.). J Integr Agr. 2021;20:120–31.
Mao TY, Liu YY, Zhu HH, Zhang J, Yang JX, Fu Q, et al. Genome-wide analyses of the bHLH gene family reveals structural and functional characteristics in the aquatic plant Nelumbo nucifera. PeerJ. 2019;7: e7153.
Sun WJ, ** X, Ma ZT, Chen H, Liu MY. Basic helix-loop-helix (bHLH) gene family in Tartary buckwheat (Fagopyrum tataricum): Genome-wide identification, phylogeny, evolutionary expansion and expression analyses. Int J Biol Macromol. 2020;155:1478–90.
Lang YH, Liu Z. Basic Helix-Loop-Helix (bHLH) transcription factor family in yellow horn (Xanthoceras sorbifolia Bunge): Genome-wide characterization, chromosome location, phylogeny, structures and expression patterns. Int J Biol Mac-romol. 2020;160:711–23.
Zhou X, Liao YL, Kim SU, Chen ZX, Nie GP, Cheng SY, et al. Genome-wide identification and characterization of bHLH family genes from Ginkgo biloba. Sci Rep. 2020;10:13723.
Xu GX, Guo CC, Shan HY, Kong HZ. Divergence of duplicate genes in exon-intron structure. P Natl Acad Sci USA. 2012;109:1187–92.
Morgenstern B, Atchley WR. Evolution of bHLH transcription factors: Modular evolution by domain shuffling? Mol Biol Evol. 1999;16:1654–63.
Kondrashov FA. Gene duplication as a mechanism of genomic adaptation to a changing environment. P Roy Soc B-Biol Sci. 2012;279:5048–57.
Adams KL, Cronn R, Percifield R, Wendel JF. Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. P Natl Acad Sci USA. 2003;100:4649–54.
Adams KL, Percifield R, Wendel JF. Organ-specific silencing of duplicated genes in a newly synthesized cotton al-lotetraploid. Genetics. 2004;168:2217–26.
Ambavaram MMR, Ali A, Ryan KP, Peoples O, Snell KD, Somleva MN. Novel transcription factors PvBMY1 and PvBMY3 increase biomass yield in greenhouse-grown switchgrass (Panicum virgatum L.). Plant Sci. 2018;273:100–9.
Testone G, Baldoni E, Iannelli MA, Nicolodi C, Di Giacomo E, Pietrini F, et al. Transcription factor networks in leaves of Cichorium endivia: New insights into the relationship between photosynthesis and leaf development. Plants-Basel. 2019;8:531.
Perveen S, Qu MN, Chen FM, Essemine J, Khan N, Lyu MJA, et al. Overexpression of maize transcription factor mEmBP-1 increases photosynthesis, biomass, and yield in rice. J Exp Bot. 2020;71:4944–57.
Feller A, Machemer K, Braun EL, Grotewold E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011;66:94–116.
Lamesch P, Berardini TZ, Li DH, Swarbreck D, Wilks C, Sasidharan R, et al. The Arabidopsis information resource (TAIR): improved gene annotation and new tools. Nucleic Acids Res. 2012;40:D1202–10.
Chen FM, Zheng GY, Qu MN, Wang YJ, Lyu MJA, Zhu XG. Knocking out NEGATIVE REGULATOR OF PHOTOSYNTHESIS 1 increases rice leaf photosynthesis and biomass production in the field. J Exp Bot. 2021;72:1836–49.
Monte E, Tepperman JM, Al-Sady B, Kaczorowski KA, Alonso JM, Ecker JR, et al. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. P Natl Acad Sci USA. 2004;101:16091–8.
Voorrips RE. MapChart: Software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93:77–8.
Wang YP, Tang HB, DeBarry JD, Tan X, Li JP, Wang XY, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40: e49.
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: An infor-mation aesthetic for comparative genomics. Genome Res. 2009;19:1639–45.
Goldman N, Yang ZH. Codon-based model of nucleotide substitution for protein-coding DNA-sequences. Mol Biol Evol. 1994;11:725–36.
Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genoty** with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907-+.
Liao Y, Smyth GK, Shi W. FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30.
Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–8.
Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, et al. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–202.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–8.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Acknowledgements
This research was financially supported by the Innovation and Technology program of Colleges and Universities in Shandong Province (grant number 2019KJF015).
Funding
This study was supported by the Innovation and Technology program of Colleges and Universities in Shandong Province (grant number 2019KJF015).
Author information
Authors and Affiliations
Contributions
Ruihua Wang designed the experiments, carried out the data analysis and wrote the manuscript draft; Yuanyuan Li designed the experiments, provided the RNA-seq data and revised the manuscript; Min Han performed the qRT-PCR and psychological experiments; Minggang Gao carried out the data analysis; Huilian Liu revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The use of plant materials in the study complies with local and national regulations. The plant materials used in the experiment were obtained from our laboratory, and no permits are required for collection of the plant materials from the big field. This article did not contain any studies with human participants or animals and did not involve any endangered or protected species.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The information of the identified bHLH genes in Raphanus sativus L. and Arabidopsis thaliana
Additional file 2: FigureS1.
Sequencelogos of 19 conserved motifs. The height of a letter indicates its relativefrequency at the given position
Additional file 3: Table S2.
The duplication type of bHLH genes in Raphanus sativus L. WGD means whole genome duplication
Additional file 4: Table S3.
Non-synonymous and synonymous substitution rates of the 48 paralogous bHLH gene pairs. dN means non-synonymous substitution ratio; dS means synonymous substitution ratio
Additional file 5: Table S4.
The list of cis-elements in RsbHLH gene promoters
Additional file 6: Table S5.
The differentially expressed RsbHLH genes between GF and WF at stage 1 to stage 5
Additional file 7: Figure S2.
The expression levels of the fifteen RsbHLHs at the fifth stage of GF and WF by qRT-PCR andRNA-seq. qRT-PCR were normalized to the expression of Actin
Additional file 8: Table S6.
The relative expression data of the fifteen RsbHLHs at the fifth stage of GF and WF by qRT-PCR.
Additional file 9: Table S7.
Primer sequences of the selected fifteen bHLH genes for qRT-PCR in Raphanus sativus L
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, R., Li, Y., Gao, M. et al. Genome-wide identification and characterization of the bHLH gene family and analysis of their potential relevance to chlorophyll metabolism in Raphanus sativus L. BMC Genomics 23, 548 (2022). https://doi.org/10.1186/s12864-022-08782-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08782-4