Abstract
Background
In the last decade, graphene oxide-based nanomaterials, such as graphene oxide (GO) and reduced graphene oxide (rGO), have attracted more and more attention in the field of biomedicine. Due to the versatile surface functionalization, ultra-high surface area, and excellent biocompatibility of graphene oxide-based nanomaterials, which hold better promise for potential applications than among other nanomaterials in biomedical fields including drug/gene delivery, biomolecules detection, tissue engineering, especially in cancer treatment.
Results
Here, we review the recent progress of graphene oxide-based multifunctional nanomaterials for cancer treatment. A comprehensive and in-depth depiction of unique property of graphene oxide-based multifunctional nanomaterials is first interpreted, with particular descriptions about the suitability for applying in cancer therapy. Afterward, recently emerging representative applications of graphene oxide-based multifunctional nanomaterials in antitumor therapy, including as an ideal carrier for drugs/genes, phototherapy, and bioimaging, are systematically summarized. Then, the biosafety of the graphene oxide-based multifunctional nanomaterials is reviewed.
Conclusions
Finally, the conclusions and perspectives on further advancing the graphene oxide-based multifunctional nanomaterials toward potential and versatile development for fundamental researches and nanomedicine are proposed.
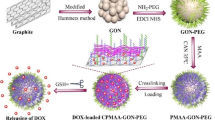
Graphic abstract

Similar content being viewed by others
Background
Cancer has always been a serious threat to human life and health, which needs to be solved urgently. Although traditional therapeutic strategies including chemotherapy together, radiotherapy, and surgery (Peer et al. 2007) have demonstrated plenty of achievements, they are still limited in clinical applications due to drawbacks like multidrug resistant (MDR) effect, poor bioavailability, and non-specific biodistribution in the body (Yi et al. 2019; Yan et al. 2021). To improve the safety and effectiveness of tumor therapy, the development of a novel anticancer therapeutic strategy becomes one of the key issues. In recent years, new methods facilitated by using nanomaterials show great promises in anticancer treatment (Qian et al. 2017; Yan et al. 2018, 2020).
As a shining star among nanomaterials, graphene oxide-based nanomaterials have gained significant research interests globally ever since it was first demonstrated in the year of 2004 (Kakran et al. 2011). In 2008, Dai’s research team first demonstrated that polyethylene glycol (PEG)-GO can be used as a versatile platform for delivering anticancer drugs delivery. The investigation of graphene oxide-based nanomaterials has opened new perspectives for cancer treatment with improved therapeutic efficiency (Liu et al. 2008).
Graphene oxide-based nanomaterials, GO and rGO, have unique property including chemical and mechanical stability, two-dimensional structures, and biocompatibility. Moreover, they have a large and easy-to-modify surfaces that can be modified to link with epoxide hydroxyl, carboxyl, and hydroxy (–O–, –COOH, –OH) groups. These groups can be further used to change the surface characteristic of GO and provide attachment sites to various molecules, including protein, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) (Yang et al. 2010) designed Fe3O4 decorated poly (4-styrene sulfonate)-GO for MRI application by the high-temperature thermal decomposition method. The prepared functional GO exhibited good water solubility and excellent MRI effect. Zhang et al. (2013) report on the development of a two-dimensional nanomaterial GO-based T1 MRI CA. Compared with gadolinium diethylene triamine pentaacetate (Gd-DTPA), gadolinium-functionalized nanographene oxide (Gd-NGO) showed a much higher T1 relaxivity value (r1) and contrast of in vivo T1-weighted MRI. Moreover, Peng et al. (2012) developed a MnFe2O4 nanoparticle decorated-GO for T2-weighted MRI, which got a high T2 relaxivity value (r2) of 256.2 (mM)−1 s−1.
The combination of MRI and PTT is of great significance to realize simultaneous diagnosis and treatment. For instance, Meng et al. (2017) prepared nanoscaled metal–organic frames (NMOFs) composited GO and used it in tumor-guided PTT with MRI. Results revealed that the fabricated NMOFs/GO was effective in imaging-guided PTT for clinical antitumor applications. T1-weighted MRI-guided PTT was also adopted for tumor therapy (Zhang et al. 2015a, b, c). The author designed a BaGdF5 and PEG-modified GO, which exhibited excellent T1-weighted imaging and photothermal conversion performance. According to the obvious contrast between tumor tissue and normal tissue on the MRI, the location and size of the tumor can be clearly obtained. Then, the tumor area of the mice treated with GO/BaGdF5/PEG was irradiated by a near-infrared laser to produced significant heating to achieve the antitumor effect.
Fluorescence imaging
FLI is a non-invasive technique based on photons emitted by fluorescent probes (Baker and Baker 2010; Zhu et al. 2013; Tang et al. Biosafety It is crucial to study the biosafety of graphene oxide-based nanomaterials in vitro and in vivo to determine whether they can be used as clinical candidates. So far, extensive studies have been illustrated the biotoxicity, immunological compatibility, immunological compatibility, and inflammatory responses of graphene oxide-based nanomaterials. Graphene oxide-based nanomaterials show great potentials in cancer treatment due to outstanding properties such as surface properties, photothermal property, and pH sensitivity. However, researchers need to consider their biotoxicity as one of the primary issues (Wang et al. 2011). Most researches have indicated that the biological toxicity of graphene oxide mainly comes from its surface properties: charge, oxygen content, surface structure, lateral dimension, and corona effect. (Guo et al. 2014). Besides, other factors are involved in influencing biotoxicity: cell types, concentration, and detection methods (Gautam et al. Miyanda and Gautam, 2017). It has been proven that graphene oxide-based nanocomposites show toxicity effects to both prokaryotic and eukaryotic cells. For toxicity to eukaryotic cells, numbers of mechanisms have been proposed, such as DNA and mitochondrial damage (Liu et al. 2013a, b, c), inflammatory responses (Orecchioni et al. 2016), autophagy (Huang et al. 2015), necrosis (Qu et al. 2013), and apoptosis (Li et al. 2012a, b). For toxicity to prokaryotic cells, Zou et al. (2016) summarized the mechanisms of the antimicrobial activities of graphene oxide-based nanomaterials, such as encapsulate and capture bacterial membranes. Furthermore, results of in vitro and in vivo experiments have shown that GO had obvious dose-dependent toxicity. In studying the toxicity of GO in mice, it was found that low-dose (0.1 mg) and medium-dose (0.25 mg) GO had almost no effect on mice. While when the dose reached 4 mg, it showed liver and lung damage (Wang et al. 2011). In addition, chromosomal aberrations and DNA damage induced by GO were also found (Durán et al. 2017). Hemocompatibility investigation is an important toxicity assessment of GO (Kiew et al. 2016). In general, fresh blood collected from healthy animals is applied for hemolytic analysis to evaluate hemocompatibility. The hemolytic properties of GO were usually caused due to the electrostatic interaction between the GO and membrane of red blood cell (RBC). However, proper surface modifications could improve the hemocompatibility of GO. For example, by adopting CS, the hemolytic activity of GO can be significantly eliminated (Wu et al. 2015). Immunological compatibility of GO is also considered as a factor of biosafety. It has been reported that the presence of GO could cause strong immunogenicity proved by a remarkable increase of tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) (Zhi et al. 2013). Meanwhile, PVP-modified GO possesses better immunological compatibility. When macrophages play the role of unconventional immune defense, GO can be eliminated or cause inflammation before reaching the target. Yue et al. (2012) found that IL-6, TNF-α, monocyte chemotactic protein 1 (MCP-1), interleukin-12 (IL-12), and interferon γ (IFN-γ) can be increased significantly in the presence of GO, leading to serious inflammatory responses. Ma et al. (2015) reported GO can induce more inflammatory cytokines by interacting with toll-like receptors to activate the NF-kappaB (NF-kB) pathway. Meanwhile, some studies showed that functionalized NGOs could avoid inflammatory responses by macrophages via weakening the opsonin–protein interaction (Kiew et al. 2016).Biotoxicity
Hemocompatibility
Immunological compatibility
Inflammatory responses
Conclusion
This review aims to investigate the applications of graphene oxide-based nanomaterials (e.g., GO and rGO) in cancer therapy including drug/gene delivery, phototherapy, bioimaging. In terms of drug and gene delivery, compared with other drug delivery systems, graphene oxide-based nanomaterials can obviously display high drug loading rate, targeting effect, increasing the sensitivity of chemotherapy drugs/genes. For the application of phototherapy, the combination of graphene oxide-based nanomaterials with PTT to achieve tumor elimination through the photothermal effect of GO. PDT based on graphene oxide-based nanomaterials can increase the water solubility of hydrophobic PSs and selectively deliver PS to cancer cells via the EPR effect. Meanwhile, the therapeutic diagnosis platform assembled by graphene oxide-based nanomaterials is applied to the early diagnosis and treatment of cancer. Besides, to obtain the best cancer treatment effect, new treatment strategies have been proposed such as PTT-PDT, chemo-PTT, and chemo-PDT.
A review of current literature has revealed the majority of present studies demonstrate that graphene oxide-based nanomaterials hold a bright future in nanomedicine. This review not only summarizes the excellent achievements in the last decades, but also pays particular attentions to the latest achievements in 2 years. Moreover, this review represents a comprehensive summary of all the aspects of applying graphene oxide-based nanomaterials in cancer treatment. Overall, this review can help readers achieve comprehensive understanding of the application of graphene oxide-based nanomaterials in tumor treatment and grasp the latest research achievements, thus inspiring novel research ideas and making contribution to the research field of graphene oxide-based nanomaterials for antitumor therapy. Unfortunately, there still exist some requirements to address the remaining challenges. One of the concerns for the challenge is the toxicity of graphene oxide-based nanomaterials. Although a large number of studies have been conducted to confirm the in vitro and in vivo toxicity of graphene oxide-based nanomaterials and its derivatives, the potential nanotoxicity requires further in-depth investigations. For in vitro toxicity, it is very important to understand its mechanism, especially the cellular uptake mechanism of graphene oxide-based nanomaterials. For in vivo toxicity, it is necessary to study the absorption, distribution, metabolism, and excretion in vivo. Furthermore, how to properly design graphene oxide-based nanomaterials to achieve the desired therapeutic effect is a critical issue. On the one hand, to diagnose and treat tumors, drugs or other functional agents need to be successfully delivered to tumor tissues and retained for a long time. Graphene oxide-based nanomaterials need to be designed to a suitable size to avoid the endoplasmic reticulation of large-size nanomaterials and the rapid clearance of ultra-small nanomaterials and to effectively passively target the tumor site. On the other hand, according to the receptors overexpressed on the tumor cell membrane to ensure the effective accumulation and retention of graphene oxide-based nanomaterials in the tumor. Endogenous and exogenous stimuli should also be fully utilized to realize the intelligent regulation of tumor nanoplatforms. Besides, many shortcomings in the design of graphene oxide-based nanomaterials as the multifunctional platform should be avoided, including complex design, cumbersome synthesis, low integration efficiency, lack of synergy, and uncertain biological responses.
In conclusion, graphene oxide-based nanomaterials have brought many surprises and challenges. In the near future graphene oxide-based nanomaterials will arouse ultimate benefits for human diseases treatment, especially for cancer treatment.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A549cells:
-
Lung adenocarcinoma cells
- AgNPs:
-
Silver nanoparticles
- AuNPs:
-
Gold nanoparticles
- anti-CD20:
-
Rituxan
- BSA:
-
Bovine serum albumin
- B-rGO:
-
Bacterially reduced graphene oxide
- Ce6:
-
Chlorin e6
- CD-44:
-
Cluster of differentiation-44
- CS:
-
Chitosan
- CT:
-
Computed tomography
- CAs:
-
Contrast agents
- CPT:
-
Camptothecin
- C255:
-
Anti-EGFR antibodies
- CAMR:
-
Cell attach molecular receptor
- DEX:
-
Dextran
- DNA:
-
Deoxyribonucleic acid
- DOX:
-
Doxorubicin hydrochloride
- EPI:
-
Epirubicin
- EGFR:
-
Epidermal growth factor receptor
- EPR:
-
Enhance permeability and retention
- FACO:
-
Folic acid-conjugated chitosan oligosaccharide
- FACO+ :
-
Folic acid-conjugated chitosan oligosaccharide containing quaternary ammonium groups
- FLimaging:
-
Fluorescence imaging
- FDA:
-
Food and Drug Administration
- FGO:
-
Fluorinated graphene oxide
- GC:
-
Galactosylated chitosan
- NGR:
-
AsnGly-Arg
- GA:
-
Glycyrrhetinic acid
- GONS:
-
GO-nanoshells
- GSH:
-
Glutathione
- GO:
-
Graphene oxide
- GOCL:
-
Graphene oxide/cationic lipid
- HA:
-
Hypocrellin A
- HCC:
-
Human hepatocellular carcinoma
- HepG2cells:
-
Human liver cancer cells
- HeLacells:
-
Human cervical cancer cells
- HEK-293:
-
Human embryonic kidney
- HA:
-
Hyaluronic acid
- IC50 :
-
Half-maximum inhibitory concentration
- ICG:
-
Indocyanine green
- IL-1:
-
Interleukin-1
- IL-6:
-
Interleukin-6
- IL-12:
-
Interleukin-12
- IFN-γ:
-
Interferon γ
- LA:
-
Lactobionic acid
- LSPR:
-
Local surface plasmon resonance
- mPEG:
-
Methoxypolyethylene glycol
- MS:
-
Mesoporous silica
- MRI:
-
Magnetic resonance imaging
- MDR:
-
Multiple drug resistance
- MB:
-
Methylene blue
- MCP-1:
-
Monocyte chemotactic protein 1
- NB:
-
O-Nitrobenzyl derivative linker
- NIR:
-
Near infrared
- NF-kB:
-
NF-kappaB
- NGO:
-
Nanographene oxide
- nGO:
-
Nanographene oxide sheet
- NPs:
-
Nanoparticles
- NMOFs:
-
Nanoscale metal–organic frames
- NIH/3T3:
-
Mouse normal fibroblast cell
- PL:
-
Photoluminescence
- PEG:
-
Polyethylene glycol
- PLL:
-
Poly-l-lysine
- PplX:
-
Protoporphyrin IX
- PVA:
-
Poly-vinylalcohol
- PF127:
-
Pluronic F127
- PEI:
-
Poly-ethylenimine
- PAA:
-
Polyacrylic acid
- PTX:
-
Paclitaxel
- pDNA:
-
Plasmid DNA
- PTT:
-
Photothermal therapy
- PDT:
-
Photodynamic therapy
- PSs:
-
Photosensitizers
- P-gp:
-
P-glycoprotein
- PVP:
-
Polyvinylpyrrolidone
- PAI:
-
Photoacoustic imaging
- PA:
-
Photoacoustic
- QY:
-
Fluorescence quantum yield
- rGO:
-
Reduced graphene oxide
- r 1 :
-
T1 Relaxivity value
- r 2 :
-
T2 Relaxivity value
- ROSs:
-
Reactive oxygen species
- RGD4C:
-
ACDCRGDCFCG peptide
- RBC:
-
Red blood cell
- RNA:
-
Ribonucleic acid
- SERS:
-
Surface-enhanced Raman scattering
- ssDNA:
-
Single-stranded DNA
- siRNA:
-
Small interfering RNA
- SA:
-
Sodium alginate
- SN38:
-
7-Ethyl-10-hydroxycamptothecin
- Tf:
-
Transferrin
- TNF-α:
-
Tumor necrosis factor alpha
- UC:
-
Up-conversion
- VEGFR:
-
Vascular endothelial growth factor receptor
- –OH:
-
Hydroxyl
- –O–:
-
Epoxide
- –COOH:
-
Carboxylic acid
References
Abed S, Bakhsheshi-Rad HR, Yaghoubi H, Ning L, Chen X. Antibacterial activities of zeolite/silver–graphene oxide nanocomposite in bone implants. Mater Technol. 2020. https://doi.org/10.1080/10667857.2020.1786784.
Aliabadi M, Shagholani H, Yunessnia Lehi A. Synthesis of a novel biocompatible nanocomposite of graphene oxide and magnetic nanoparticles for drug delivery. Int J Biol Macromol. 2017;98:287–91. https://doi.org/10.1016/j.ijbiomac.2017.02.012.
Alibolandi M, Mohammadi M, Taghdisi SM, Ramezani M, Abnous K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohydr Polym. 2017;155:218–29. https://doi.org/10.1016/j.carbpol.2016.08.046.
Bai H, Li C, Shi G. Functional composite materials based on chemically converted graphene. Adv Mater. 2011;23(9):1089–115. https://doi.org/10.1002/adma.201003753.
Bai LY, Yang XQ, An J, Zhang L, Zhao K, Qin MY, Fang BY, Li C, Xuan Y, Zhang XS, Zhao YD, Ma ZY. Multifunctional magnetic-hollow gold nanospheres for bimodal cancer cell imaging and photothermal therapy. Nanotechnology. 2015;26(31): 315701. https://doi.org/10.1088/0957-4484/26/31/315701.
Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angew Chem. 2010;49(38):6726–44. https://doi.org/10.1002/anie.200906623 (International ed. in English).
Bao T, Yin W, Zheng X, Zhang X, Yu J, Dong X, Yong Y, Gao F, Yan L, Gu Z, Zhao Y. One-pot synthesis of PEGylated plasmonic MoO(3-x) hollow nanospheres for photoacoustic imaging guided chemo-photothermal combinational therapy of cancer. Biomaterials. 2016;76:11–24. https://doi.org/10.1016/j.biomaterials.2015.10.048.
Brodie BC. On the atomic weight of graphite. Proc R Soc Lond. 1859;10:11–2. https://doi.org/10.1098/rspl.1859.0007.
Byun E, Lee H. Enhanced loading efficiency and sustained release of doxorubicin from hyaluronic acid/graphene oxide composite hydrogels by a mussel-inspired catecholamine. J Nanosci Nanotechnol. 2014;14(10):7395–401. https://doi.org/10.1166/jnn.2014.9571.
Cao X, Feng F, Wang Y, Yang X, Duan H, Chen Y. Folic acid-conjugated graphene oxide as a transporter of chemotherapeutic drug and sirna for reversal of cancer drug resistance. J Nanopart Res. 2013;15(10):1965. https://doi.org/10.1007/s11051-013-1965-y.
Cao L, Zhang F, Wang Q, Wu X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;79:697–701. https://doi.org/10.1016/j.msec.2017.05.056.
Chang JE, Cho HJ, Jheon S. Anticancer efficacy of photodynamic therapy with lung cancer-targeted nanoparticles. J vis Exp. 2016;118:54865. https://doi.org/10.3791/54865.
Chen W, Zhang J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J Nanosci Nanotechnol. 2006;6(4):1159–66. https://doi.org/10.1166/jnn.2006.327.
Chen JT, Fu YJ, An QF, Lo SC, Huang SH, Hung WS, Hu CC, Lee KR, Lai JY. Tuning nanostructure of graphene oxide/polyelectrolyte LbL assemblies by controlling pH of GO suspension to fabricate transparent and super gas barrier films. Nanoscale. 2013a;5(19):9081–8. https://doi.org/10.1039/c3nr02845c.
Chen Y, Qi Y, Liu B. Polyacrylic acid functionalized nanographene as a nanocarrier for loading and controlled release of doxorubicin hydrochloride. J Nanomater. 2013b;2013(5):3805–16. https://doi.org/10.1155/2013/345738.
Chen GY, Chen CL, Tuan HY, Yuan PX, Li KC, Yang HJ, Hu YC. Graphene oxide triggers toll-like receptors/autophagy responses in vitro and inhibits tumor growth in vivo. Adv Healthc Mater. 2014a;3(9):1486–95. https://doi.org/10.1002/adhm.201300591.
Chen H, Wang Z, Zong S, Wu L, Chen P, Zhu D, Wang C, Xu S, Cui Y. SERS-fluorescence monitored drug release of a redox-responsive nanocarrier based on graphene oxide in tumor cells. ACS Appl Mater Interfaces. 2014b;6(20):17526–33. https://doi.org/10.1021/am505160v.
Chen J, Wang X, Chen T. Facile and green reduction of covalently PEGylated nanographene oxide via a ‘water-only’ route for high-efficiency photothermal therapy. Nanoscale Res Lett. 2014c;9(1):86. https://doi.org/10.1186/1556-276X-9-86.
Chen J, Liu C, Zeng G, You Y, Wang H, Gong X, Zheng R, Kim J, Kim C, Song L. Indocyanine green loaded reduced graphene oxide for in vivo photoacoustic/fluorescence dual-modality tumor imaging. Nanoscale Res Lett. 2016;11(1):85. https://doi.org/10.1186/s11671-016-1288-x.
Chen L, Wang Y, Song J, Deng W, Lu J, Ma L, Yang C, Li M, Xue Y. Phosphoproteome-based kinase activity profiling reveals the critical role of MAP2K2 and PLK1 in neuronal autophagy. Autophagy. 2017a;13(11):1969–80. https://doi.org/10.1080/15548627.2017.1371393.
Chen Y, Zhang F, Wang Q, Tong R, Lin H, Qu F. Near-infrared light-mediated LA–UCNPs@SiO2–C/HA@mSiO2–DOX@NB nanocomposite for chemotherapy/PDT/PTT and imaging. Dalton Trans. 2017b;46(41):14293–300. https://doi.org/10.1039/c7dt02529g.
Chowdhury SM, Surhland C, Sanchez Z, Chaudhary P, Suresh Kumar MA, Lee S, Peña LA, Waring M, Sitharaman B, Naidu M. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomed Nanotechnol Biol Med. 2015;11(1):109–18. https://doi.org/10.1016/j.nano.2014.08.001.
Chua CK, Pumera M. Covalent chemistry on graphene. Chem Soc Rev. 2013;42(8):3222–33. https://doi.org/10.1039/c2cs35474h.
Chung C, Kim YK, Shin D, Ryoo SR, Min DH. Biomedical applications of graphene and graphene oxide. Acc Chem Res. 2013;46(10):2211–24. https://doi.org/10.1021/ar300159f.
Cong HP, He JJ, Lu Y, Yu SH. Water-soluble magnetic-functionalized reduced graphene oxide sheets: in situ synthesis and magnetic resonance imaging applications. Small. 2010;6(2):169–73. https://doi.org/10.1002/smll.200901360.
Denkova AG, de Kruijff RM, Serra-Crespo P. Nanocarrier-mediated photochemotherapy and photoradiotherapy. Adv Healthc Mater. 2018;7(8): e1701211. https://doi.org/10.1002/adhm.201701211.
Desbrières J, Martinez C, Rinaudo M. Hydrophobic derivatives of chitosan: characterization and rheological behaviour. Int J Biol Macromol. 1996;19(1):21–8. https://doi.org/10.1016/0141-8130(96)01095-1.
Di Santo R, Quagliarini E, Palchetti S, Pozzi D, Palmieri V, Perini G. Microfluidic-generated lipid-graphene oxide nanoparticles for gene delivery. Appl Phys Lett. 2019;114(23):233701.1-233701.5. https://doi.org/10.1063/1.5100932.
Díez-Pascual AM, Díez-Vicente AL. Poly(propylene fumarate)/polyethylene glycol-modified graphene oxide nanocomposites for tissue engineering. ACS Appl Mater Interfaces. 2016;8(28):17902–14. https://doi.org/10.1021/acsami.6b05635.
Dinescu S, Ionita M, Ignat SR, Costache M, Hermenean A. Graphene oxide enhances chitosan-based 3D scaffold properties for bone tissue engineering. Int J Mol Sci. 2019;20(20):5077. https://doi.org/10.3390/ijms20205077.
Ding Y, Zhou L, Chen X, Wu Q, Song Z, Wei S, Zhou J, Shen J. Mutual sensitization mechanism and self-degradation property of drug delivery system for in vitro photodynamic therapy. Int J Pharm. 2016;498(1–2):335–46. https://doi.org/10.1016/j.ijpharm.2015.12.044.
Dong H, Zhao Z, Wen H, Guo F, Shen A, Pilger F, Lin C, Shi D. Poly (ethylene glycol) conjugated nano-graphene oxide for photodynamic therapy. Sci China Chem. 2010;53(011):2265–71. https://doi.org/10.1007/s11426-010-4114-9.
Dou R, Du Z, Bao T, Dong X, Zheng X, Yu M, Yin W, Dong B, Yan L, Gu Z. The polyvinylpyrrolidone functionalized rGO/Bi2S3 nanocomposite as a near-infrared light-responsive nanovehicle for chemo-photothermal therapy of cancer. Nanoscale. 2016;8(22):11531–42. https://doi.org/10.1039/c6nr01543c.
Dreaden EC, Mackey MA, Huang X, Kang B, El-Sayed MA. Beating cancer in multiple ways using nanogold. Chem Soc Rev. 2011;40(7):3391–404. https://doi.org/10.1039/c0cs00180e.
Du L, Wu S, Li Y, Zhao X, Ju X, Wang Y. Cytotoxicity of PEGylated graphene oxide on lymphoma cells. Bio-Med Mater Eng. 2014;24(6):2135–41. https://doi.org/10.3233/BME-141024.
Du B, Liu J, Ding G, Han X, Li D, Wang E. Positively charged graphene/Fe3O4/polyethylenimine with enhanced drug loading and cellular uptake for magnetic resonance imaging and magnet-responsive cancer therapy. Nano Res. 2017;10(007):2280–95. https://doi.org/10.1007/S12274-016-1418-X.
Du S, Wang Y, Ao J, Wang K, Zhang Z, Yang L. Targeted delivery of sirna to ovarian cancer cells using functionalized graphene oxide. Nano Life. 2018. https://doi.org/10.1142/S1793984418500010.
Durán N, Martinez DS, Silveira CP, Durán M, de Moraes AC, Simões MB, Alves OL, Fávaro WJ. Graphene oxide: a carrier for pharmaceuticals and a scaffold for cell interactions. Curr Top Med Chem. 2015;15(4):309–27. https://doi.org/10.2174/1568026615666150108144217.
Durán M, Durán N, Fávaro WJ. In vivo nanotoxicological profile of graphene oxide. J Phys Conf Ser. 2017;838(1):012026. https://doi.org/10.1088/1742-6596/838/1/012026.
Ege D, Kamali A, Boccaccini A. Graphene oxide/polymer-based biomaterials. Adv Eng Mater. 2017;19:1700627. https://doi.org/10.1002/adem.201700627.
Fan L, Ge H, Zou S, **ao Y, Wen H, Li Y, Feng H, Nie M. Sodium alginate conjugated graphene oxide as a new carrier for drug delivery system. Int J Biol Macromol. 2016;93(Pt A):582–90. https://doi.org/10.1016/j.ijbiomac.2016.09.026.
Feng L, Yang X, Shi X, Tan X, Peng R, Wang J, Liu Z. Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small. 2013;9(11):1989–97. https://doi.org/10.1002/smll.201202538.
Gao L, Yu J, Liu Y, Zhou J, Sun L, Wang J, Zhu J, Peng H, Lu W, Yu L, Yan Z, Wang Y. Tumor-penetrating peptide conjugated and doxorubicin loaded T1–T2 dual mode MRI contrast agents nanoparticles for tumor theranostics. Theranostics. 2018;8(1):92–108. https://doi.org/10.7150/thno.21074.
Gao Y, Ma Q, Cao J, Wang Y, Yang X, Xu Q, Liang Q, Sun Y. Recent advances in microfluidic-aided chitosan-based multifunctional materials for biomedical applications. Int J Pharm. 2021;600: 120465. https://doi.org/10.1016/j.ijpharm.2021.120465.
Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, Hobza P, Zboril R, Kim KS. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev. 2012;112(11):6156–214. https://doi.org/10.1021/cr3000412.
Ghosh S, Chatterjee K. Poly (ethylene glycol) functionalized graphene oxide in tissue engineering: a review on recent advances. Int J Nanomed. 2020;15:5991–6006. https://doi.org/10.2147/IJN.S249717.
Gollavelli G, Ling YC. Multi-functional graphene as an in vitro and in vivo imaging probe. Biomaterials. 2012;33(8):2532–45. https://doi.org/10.1016/j.biomaterials.2011.12.010.
Gong P, Ji S, Wang J, Dai D, Wang F, Tian M. Fluorescence-switchable ultrasmall fluorinated graphene oxide with high near-infrared absorption for controlled and targeted drug delivery. Chem Eng J. 2018;348:438–46. https://doi.org/10.1016/j.cej.2018.04.193.
Guo X, Huang L. Recent advances in nonviral vectors for gene delivery. Acc Chem Res. 2012;45(7):971–9. https://doi.org/10.1021/ar200151m.
Guo X, Mei N. Assessment of the toxic potential of graphene family nanomaterials. J Food Drug Anal. 2014;22(1):105–15. https://doi.org/10.1016/j.jfda.2014.01.009.
Guo M, Mao H, Li Y, Zhu A, He H, Yang H, Wang Y, Tian X, Ge C, Peng Q, Wang X, Yang X, Chen X, Liu G, Chen H. Dual imaging-guided photothermal/photodynamic therapy using micelles. Biomaterials. 2014;35(16):4656–66. https://doi.org/10.1016/j.biomaterials.2014.02.018.
Gurunathan S, Han JW, Eppakayala V, Kim JH. Green synthesis of graphene and its cytotoxic effects in human breast cancer cells. Int J Nanomed. 2013;8:1015–27. https://doi.org/10.2147/IJN.S42047.
Gurunathan S, Han JW, Kim ES, Park JH, Kim JH. Reduction of graphene oxide by resveratrol: a novel and simple biological method for the synthesis of an effective anticancer nanotherapeutic molecule. Int J Nanomed. 2015;10:2951–69. https://doi.org/10.2147/IJN.S79879.
Han H, Wang H, Chen Y, Li Z, Wang Y, ** Q, Ji J. Theranostic reduction-sensitive gemcitabine prodrug micelles for near-infrared imaging and pancreatic cancer therapy. Nanoscale. 2016;8(1):283–91. https://doi.org/10.1039/c5nr06734k.
He S, Song J, Qu J, Cheng Z. Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics. Chem Soc Rev. 2018;47(12):4258–78. https://doi.org/10.1039/c8cs00234g.
Hoseini-Ghahfarokhi M, Mirkiani S, Mozaffari N, Abdolahi Sadatlu MA, Ghasemi A, Abbaspour S, Akbarian M, Farjadain F, Karimi M. Applications of graphene and graphene oxide in smart drug/gene delivery: is the world still flat? Int J Nanomed. 2020;15:9469–96. https://doi.org/10.2147/IJN.S265876.
Hosseinzadeh R, Khorsandi K, Hosseinzadeh G. Graphene oxide-methylene blue nanocomposite in photodynamic therapy of human breast cancer. J Biomol Struct Dyn. 2018;36(9):2216–23. https://doi.org/10.1080/07391102.2017.1345698.
Hu D, Zhang J, Gao G, Sheng Z, Cui H, Cai L. Indocyanine green-loaded polydopamine-reduced graphene oxide nanocomposites with amplifying photoacoustic and photothermal effects for cancer theranostics. Theranostics. 2016;6(7):1043–52. https://doi.org/10.7150/thno.14566.
Huang P, Lin J, Wang S, Zhou Z, Li Z, Wang Z, Zhang C, Yue X, Niu G, Yang M, Cui D, Chen X. Photosensitizer-conjugated silica-coated gold nanoclusters for fluorescence imaging-guided photodynamic therapy. Biomaterials. 2013;34(19):4643–54. https://doi.org/10.1016/j.biomaterials.2013.02.063.
Huang P, Rong P, Lin J, Li W, Yan X, Zhang MG, Nie L, Niu G, Lu J, Wang W, Chen X. Triphase interface synthesis of plasmonic gold bellflowers as near-infrared light mediated acoustic and thermal theranostics. J Am Chem Soc. 2014;136(23):8307–13. https://doi.org/10.1021/ja503115n.
Huang P, Wang S, Wang X, Shen G, Lin J, Wang Z, Guo S, Cui D, Yang M, Chen X. Surface functionalization of chemically reduced graphene oxide for targeted photodynamic therapy. J Biomed Nanotechnol. 2015;11(1):117–25. https://doi.org/10.1166/jbn.2015.2055.
Hummers WSH Jr, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958. https://doi.org/10.1021/ja01539a017?journalCode=jacsat.
Jiang W, Mo F, Lin Y, Wang X, Xu L, Fu F. Tumor targeting dual stimuli responsive controllable release nanoplatform based on DNA-conjugated reduced graphene oxide for chemo-photothermal synergetic cancer therapy. J Mater Chem B. 2018;6(26):4360–7. https://doi.org/10.1039/c8tb00670a.
Jichlinski P, Leisinger HJ. Photodynamic therapy in superficial bladder cancer: past, present and future. Urol Res. 2001;29(6):396–405. https://doi.org/10.1007/s002400100215.
Kakran M, Sahoo NG, Bao H, Pan Y, Li L. Functionalized graphene oxide as nanocarrier for loading and delivery of ellagic acid. Curr Med Chem. 2011;18(29):4503–12. https://doi.org/10.2174/092986711797287548.
Kalluru P, Vankayala R, Chiang CS, Hwang KC. Nano-graphene oxide-mediated in vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials. 2016;95:1–10. https://doi.org/10.1016/j.biomaterials.2016.04.006.
Karlický F, Kumara Ramanatha Datta K, Otyepka M, Zbořil R. Halogenated graphenes: rapidly growing family of graphene derivatives. ACS Nano. 2013;7(8):6434–64. https://doi.org/10.1021/nn4024027.
Khdair A, Handa H, Mao G, Panyam J. Nanoparticle-mediated combination chemotherapy and photodynamic therapy overcomes tumor drug resistance in vitro. Eur J Pharm Biopharm. 2009;71(2):214–22. https://doi.org/10.1016/j.ejpb.2008.08.017.
Kiew SF, Kiew LV, Lee HB, Imae T, Chung LY. Assessing biocompatibility of graphene oxide-based nanocarriers: a review. J Control Release. 2016;226:217–28. https://doi.org/10.1016/j.jconrel.2016.02.015.
Kim H, Kim WJ. Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small. 2014;10(1):117–26. https://doi.org/10.1002/smll.201202636.
Kim H, Namgung R, Singha K, Oh IK, Kim WJ. Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug Chem. 2011a;22(12):2558–67. https://doi.org/10.1021/bc200397j.
Kim YK, Kim MH, Min DH. Biocompatible reduced graphene oxide prepared by using dextran as a multifunctional reducing agent. Chem Commun. 2011b;47(11):3195–231. https://doi.org/10.1039/c0cc05005a.
Komisar DA, Krivova GM, Stebunov YV, Yakubovsky DI, Volkov VS. Optical properties of thin graphene oxide films and their biosensing applications. J Phys: Conf Ser. 2020;1461: 012068. https://doi.org/10.1088/1742-6596/1461/1/012068.
Lalwani G, Cai X, Nie L, Wang LV, Sitharaman B. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography. Photoacoustics. 2013;1(3–4):62–7. https://doi.org/10.1016/j.pacs.2013.10.001.
Lee N, Choi SH, Hyeon T. Nano-sized CT contrast agents. Adv Mater. 2013;25(19):2641–60. https://doi.org/10.1002/adma.201300081.
Lee JY, Termsarasab U, Park JH, Lee SY, Ko SH, Shim JS, Chung SJ, Cho HJ, Kim DD. Dual CD44 and folate receptor-targeted nanoparticles for cancer diagnosis and anticancer drug delivery. J Control Release. 2016;236:38–46. https://doi.org/10.1016/j.jconrel.2016.06.021.
Li Y, Lu W, Huang Q, Huang M, Li C, Chen W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine. 2010;5(8):1161–71. https://doi.org/10.2217/nnm.10.85.
Li M, Yang X, Ren J, Qu K, Qu X. Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer’s disease. Adv Mater. 2012a;24(13):1722–8. https://doi.org/10.1002/adma.201104864.
Li Y, Liu Y, Fu Y, Wei T, Le Guyader L, Gao G, Liu RS, Chang YZ, Chen C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials. 2012b;33(2):402–11. https://doi.org/10.1016/j.biomaterials.2011.09.091.
Li JL, Tang B, Yuan B, Sun L, Wang XG. A review of optical imaging and therapy using nanosized graphene and graphene oxide. Biomaterials. 2013;34(37):9519–34. https://doi.org/10.1016/j.biomaterials.2013.08.066.
Li Y, Feng L, Shi X, Wang X, Yang Y, Yang K, Liu T, Yang G, Liu Z. Surface coating-dependent cytotoxicity and degradation of graphene derivatives: towards the design of non-toxic, degradable nano-graphene. Small. 2014;10(8):1544–54. https://doi.org/10.1002/smll.201303234.
Li Y, Dong H, Li Y, Shi D. Graphene-based nanovehicles for photodynamic medical therapy. Int J Nanomed. 2015;10:2451–9. https://doi.org/10.2147/IJN.S68600.
Li T, Liu H, ** G, Pang Y, Wu L, Wang X, Chen T. One-step reduction and PEIylation of PEGylated nanographene oxide for highly efficient chemo-photothermal therapy. J Mater Chem B. 2016;4(17):2972–83. https://doi.org/10.1039/c6tb00486e.
Li Q, Hong L, Li H, Liu C. Graphene oxide-fullerene C60 (GO–C60) hybrid for photodynamic and photothermal therapy triggered by near-infrared light. Biosens Bioelectron. 2017;89(Pt 1):477–82. https://doi.org/10.1016/j.bios.2016.03.072.
Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–74. https://doi.org/10.1038/s41571-020-0410-2.
Lim JH, Kim DE, Kim EJ, Ahrberg CD, Chung BG. Functional graphene oxide-based nanosheets for photothermal therapy. Macromol Res. 2018;26:557–65. https://doi.org/10.1007/s13233-018-6067-3.
Lin J, Chen X, Huang P. Graphene-based nanomaterials for bioimaging. Adv Drug Deliv Rev. 2016;105:242–54. https://doi.org/10.1016/j.addr.2016.05.013.
Lin W, Zhang X, Qian L, Yao N, Pan Y, Zhang L. Doxorubicin-loaded unimolecular micelle-stabilized gold nanoparticles as a theranostic nanoplatform for tumor-targeted chemotherapy and computed tomography imaging. Biomacromol. 2017;18(12):3869–80. https://doi.org/10.1021/acs.biomac.7b00810.
Lin D, Wu Z, Huang Y, Wu J, Li C, Qin W. Physical, mechanical, structural and antibacterial properties of polyvinyl alcohol/oregano oil/graphene oxide composite films. J Polym Environ. 2020;28(2):638–46. https://doi.org/10.1007/s10924-019-01627-4.
Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130(33):10876–7. https://doi.org/10.1021/ja803688x.
Liu K, Zhang JJ, Cheng FF, Zheng TT, Zhu JJ. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J Mater Chem. 2011;21(32):12034–40. https://doi.org/10.1039/C1JM10749F.
Liu Z, Guo Z, Zhong H, Qin X, Wan M, Yang B. Graphene oxide based surface-enhanced Raman scattering probes for cancer cell imaging. Phys Chem Chem Phys. 2013a;15(8):2961–6. https://doi.org/10.1039/c2cp43715e.
Liu J, Cui L, Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013b;9(12):9243–57. https://doi.org/10.1016/j.actbio.2013.08.016.
Liu Y, Luo Y, Wu J, Wang Y, Yang X, Yang R, Wang B, Yang J, Zhang N. Graphene oxide can induce in vitro and in vivo mutagenesis. Sci Rep. 2013c;3:3469. https://doi.org/10.1038/srep03469.
Liu T, Wang C, Cui W, Gong H, Liang C, Shi X, Li Z, Sun B, Liu Z. Combined photothermal and photodynamic therapy delivered by PEGylated MoS2 nanosheets. Nanoscale. 2014;6(19):11219–25. https://doi.org/10.1039/c4nr03753g.
Liu G, Qin H, Amano T, Murakami T, Komatsu N. Direct fabrication of the graphene-based composite for cancer phototherapy through graphite exfoliation with a photosensitizer. ACS Appl Mater Interfaces. 2015a;7(42):23402–6. https://doi.org/10.1021/acsami.5b07432.
Liu L, Wei Y, Zhai S, Chen Q, **ng D. Dihydroartemisinin and transferrin dual-dressed nano-graphene oxide for a pH-triggered chemotherapy. Biomaterials. 2015b;62:35–46. https://doi.org/10.1016/j.biomaterials.2015.05.036.
Liu Y, Fang N, Liu B, Song L, Wen B, Yang D. Aligned porous chitosan/graphene oxide scaffold for bone tissue engineering. Mater Lett. 2018;233(15):78–81. https://doi.org/10.1016/j.matlet.2018.08.108.
Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–108. https://doi.org/10.1039/c8cs00618k.
Loutfy SA, Salaheldin TA, Ramadan MA, Farroh K, Abdallah ZF, Youssef T. Synthesis, characterization and cytotoxic evaluation of graphene oxide nanosheets: in vitro liver cancer model. Asian Pac J Cancer Prev. 2017;18(4):955–61. https://doi.org/10.22034/APJCP.2017.18.4.955.
Lu CH, Yang HH, Zhu CL, Chen X, Chen GN. A graphene platform for sensing biomolecules. Angew Chem. 2009;48(26):4785–7. https://doi.org/10.1002/anie.200901479 (International ed. in English).
Lu N, Wang L, Lv M, Tang Z, Fan C. Graphene-based nanomaterials in biosystems. Nano Res. 2019;12(2):247–64. https://doi.org/10.1007/s12274-018-2209-3.
Lv Y, Tao L, Annie Bligh SW, Yang H, Pan Q, Zhu L. Targeted delivery and controlled release of doxorubicin into cancer cells using a multifunctional graphene oxide. Mater Sci Eng C Mater Biol Appl. 2016;59:652–60. https://doi.org/10.1016/j.msec.2015.10.065.
Ma J, Zhang J, **ong Z, Yong Y, Zhao XS. Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J Mater Chem. 2011;21(10):3350–2. https://doi.org/10.1039/c0jm02806a.
Ma J, Liu R, Wang X, Liu Q, Chen Y, Valle RP, Zuo YY, **a T, Liu S. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano. 2015;9(10):10498–515. https://doi.org/10.1021/acsnano.5b04751.
Ma N, Liu J, He W, Li Z, Luan Y, Song Y, Garg S. Folic acid-grafted bovine serum albumin decorated graphene oxide: an efficient drug carrier for targeted cancer therapy. J Colloid Interface Sci. 2017;490:598–607. https://doi.org/10.1016/j.jcis.2016.11.097.
Ma Q, Gao Y, Sun W, Cao J, Liang Y, Han S, Wang X, Sun Y. Self-assembled chitosan/phospholipid nanoparticles: from fundamentals to preparation for advanced drug delivery. Drug Deliv. 2020a;27(1):200–15. https://doi.org/10.1080/10717544.2020.1716878.
Ma Q, Cao J, Gao Y, Han S, Liang Y, Zhang T, Wang X, Sun Y. Microfluidic-mediated nano-drug delivery systems: from fundamentals to fabrication for advanced therapeutic applications. Nanoscale. 2020b;12:15512–27. https://doi.org/10.1039/d0nr02397c.
Ma Q, Song Y, Sun W, Cao J, Yuan H, Wang X, Sun Y, Shum HC. Cell-inspired all-aqueous microfluidics: from intracellular liquid–liquid phase separation toward advanced biomaterials. Adv Sci. 2020c;7(7):31. https://doi.org/10.1002/advs.201903359.
Ma Q, Haixia Ma H, Xu F, Wang X, Sun W. Microfluidics in cardiovascular disease research: state of the art and future outlook. Microsyst Nanoeng. 2021;7(1):19. https://doi.org/10.1038/s41378-021-00245-2.
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM. Improved synthesis of graphene oxide. ACS Nano. 2010;4(8):4806–14. https://doi.org/10.1021/nn1006368.
Mattheolabakis G, Milane L, Singh A, Amiji MM. Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. J Drug Target. 2015;23(7–8):605–18. https://doi.org/10.3109/1061186X.2015.1052072.
Meng J, Chen X, Tian Y, Li Z, Zheng Q. Nanoscale metal–organic frameworks decorated with graphene oxide for magnetic resonance imaging guided photothermal therapy. Chemistry. 2017;23(69):17521–30. https://doi.org/10.1002/chem.201702573.
Menon JU, Jadeja P, Tambe P, Vu K, Yuan B, Nguyen KT. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics. 2013;3(3):152–66. https://doi.org/10.7150/thno.5327.
Miyanda PM, Gautam S. Graphene oxide: a potential drug carrier for cancer therapy—review. Res Rev J Pharm Sci. 2017;8(3):21–31.
Moon H, Kumar D, Kim H, Sim C, Chang JH, Kim JM, Kim H, Lim DK. Amplified photoacoustic performance and enhanced photothermal stability of reduced graphene oxide coated gold nanorods for sensitive photoacoustic imaging. ACS Nano. 2015;9(3):2711–9. https://doi.org/10.1021/nn506516p.
Mousavi SM, Low FW, Hashemi SA, Samsudin NA, Goh SM. Development of hydrophobic reduced graphene oxide as new approach efficient photochemotherapy. RSC Adv. 2020;10(22):12851–63. https://doi.org/10.1039/d0ra00186d.
Mu L, Gao Y, Hu X. l-Cysteine: a biocompatible, breathable and beneficial coating for graphene oxide. Biomaterials. 2015;52:301–11. https://doi.org/10.1016/j.biomaterials.2015.02.046.
Muoz R, Singh DP, Kumar R, Matsuda A. Graphene oxide for drug delivery and cancer therapy. In: Nanostructured polymer composites for biomedical applications. Cambridge: Elsevier; 2019. p. 447–88. https://doi.org/10.1016/B978-0-12-816771-7.00023-5.
Muroya T, Suehiro Y, Umayahara K, Akiya T, Iwabuchi H, Sakunaga H, Sakamoto M, Sugishita T, Ten** Y. Gan to kagaku ryoho. Cancer Chemother. 1996;23(1):47–56.
Nascimento TL, Hillaireau H, Vergnaud J, Fattal E. Lipid-based nanosystems for CD44 targeting in cancer treatment: recent significant advances, ongoing challenges and unmet needs. Nanomedicine. 2016;11(14):1865–87. https://doi.org/10.2217/nnm-2016-5000.
Ocsoy I, Isiklan N, Cansiz S, Özdemir N, Tan W. ICG-conjugated magnetic graphene oxide for dual photothermal and photodynamic therapy. RSC Adv. 2016;6(36):30285–92. https://doi.org/10.1039/C6RA0679.
Olad A, Hagh K. Graphene oxide and amin-modified graphene oxide incorporated chitosan–gelatin scaffolds as promising materials for tissue engineering. Compos B Eng. 2019;162(1):692–702. https://doi.org/10.1016/j.compositesb.2019.01.040.
Orecchioni M, Ménard-Moyon C, Delogu LG, Bianco A. Graphene and the immune system: challenges and potentiality. Adv Drug Deliv Rev. 2016;105:163–75. https://doi.org/10.1016/j.addr.2016.05.014.
Pang J, Kang Z, Wang R, Xu B, Nie X, Fan L, Zhang F, Du X, Feng S, Sun D. Exploring the sandwich antibacterial membranes based on uio-66/graphene oxide for forward osmosis performance. Carbon. 2019;144:321–32. https://doi.org/10.1016/j.carbon.2018.12.050.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–60. https://doi.org/10.1038/nnano.2007.387.
Peng E, Choo ES, Chandrasekharan P, Yang CT, Ding J, Chuang KH, Xue JM. Synthesis of manganese ferrite/graphene oxide nanocomposites for biomedical applications. Small. 2012;8(23):3620–30. https://doi.org/10.1002/smll.201201427.
Poinard B, Neo S, Yeo E, Heng H, Neoh KG, Kah J. Polydopamine nanoparticles enhance drug release for combined photodynamic and photothermal therapy. ACS Appl Mater Interfaces. 2018;10(25):21125–36. https://doi.org/10.1021/acsami.8b04799.
Qian X, Gu Z, Chen Y. Two-dimensional black phosphorus nanosheets for theranostic nanomedicine. Mater Horiz. 2017;4:800–16. https://doi.org/10.1039/C7MH00305F.
Qiao Z, Zhang H, Wang K, Zhang Y. A highly sensitive and responsive fluorescent probe based on 6-azidechroman dye for detection and imaging of hydrogen sulfide in cells. Talanta. 2019;195:850–6. https://doi.org/10.1016/j.talanta.2018.12.014.
Qu G, Liu S, Zhang S, Wang L, Wang X, Sun B, Yin N, Gao X, **a T, Chen JJ, Jiang GB. Graphene oxide induces toll-like receptor 4 (TLR4)-dependent necrosis in macrophages. ACS Nano. 2013;7(7):5732–45. https://doi.org/10.1021/nn402330b.
Qu Y, He F, Yu C, Liang X, Liang D, Ma L, Zhang Q, Lv J, Wu J. Advances on graphene-based nanomaterials for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2018;90:764–80. https://doi.org/10.1016/j.msec.2018.05.018.
Ramalingam M, Wang X, Chen G, Ma P, Cui FZ. The emerging applications of graphene oxide and graphene in tissue engineering. Wiley; 2013.
Rao Z, Ge H, Liu L, Zhu C, Min L, Liu M, Fan L, Li D. Carboxymethyl cellulose modified graphene oxide as pH-sensitive drug delivery system. Int J Biol Macromol. 2018;107:1184–92. https://doi.org/10.1016/j.ijbiomac.2017.09.096.
Rosenthal A, Mantz A, Nguyen A, Bittrich E, Schubert E, Schubert M, Stamm M, Pannier AK, Uhlmann P. Biofunctionalization of titanium substrates using nanoscale polymer brushes with cell adhesion peptides. J Phys Chem B. 2018;122(25):6543–50. https://doi.org/10.1021/acs.jpcb.8b02407.
Sahu A, Choi WI, Lee JH, Tae G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials. 2013;34(26):6239–48. https://doi.org/10.1016/j.biomaterials.2013.04.066.
Shang H, Han D, Ma M, Li S, Xue W, Zhang A. Enhancement of the photokilling effect of TiO2 in photodynamic therapy by conjugating with reduced graphene oxide and its mechanism exploration. J Photochem Photobiol B Biol. 2017;177:112–23. https://doi.org/10.1016/j.jphotobiol.2017.10.016.
Sharma H, Mondal S. Functionalized graphene oxide for chemotherapeutic drug delivery and cancer treatment: a promising material in nanomedicine. Int J Mol Sci. 2020;21(17):6280. https://doi.org/10.3390/ijms21176280.
Sharman WM, Allen CM, van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today. 1999;4(11):507–17. https://doi.org/10.1016/s1359-6446(99)01412-9.
Shen H, Zhang L, Liu M, Zhang Z. Biomedical applications of graphene. Theranostics. 2012;2(3):283–94. https://doi.org/10.7150/thno.3642.
Shen Z, Ma Q, Zhou X, Zhang G, Hao G, Sun Y, Cao J. Strategies to improve photodynamic therapy efficacy by relieving the tumor hypoxia environment. NPG Asia Mater. 2021;13:39. https://doi.org/10.1038/s41427-021-00303-1.
Sheng Z, Song L, Zheng J, Hu D, He M, Zheng M, Gao G, Gong P, Zhang P, Ma Y, Cai L. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials. 2013;34(21):5236–43. https://doi.org/10.1016/j.biomaterials.2013.03.090.
Shi S, Yang K, Hong H, Valdovinos HF, Nayak TR, Zhang Y, Theuer CP, Barnhart TE, Liu Z, Cai W. Tumor vasculature targeting and imaging in living mice with reduced graphene oxide. Biomaterials. 2013;34(12):3002–9. https://doi.org/10.1016/j.biomaterials.2013.01.047.
Shi J, Wang L, Zhang J, Ma R, Gao J, Liu Y, Zhang C, Zhang Z. A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials. 2014;35(22):5847–61. https://doi.org/10.1016/j.biomaterials.2014.03.042.
Shin YC, Kim J, Kim SE, Song SJ, Hong SW, Oh JW, Lee J, Park JC, Hyon SH, Han DW. RGD peptide and graphene oxide co-functionalized PLGA nanofiber scaffolds for vascular tissue engineering. Regener Biomater. 2017;4(3):159–66. https://doi.org/10.1093/rb/rbx001.
Singh DP, Herrera CE, Singh B, Singh S, Singh RK, Kumar R. Graphene oxide: an efficient material and recent approach for biotechnological and biomedical applications. Mater Sci Eng C Mater Biol Appl. 2018;86:173–97. https://doi.org/10.1016/j.msec.2018.01.004.
Song E, Han W, Li C, Cheng D, Li L, Liu L, Zhu G, Song Y, Tan W. Hyaluronic acid-decorated graphene oxide nanohybrids as nanocarriers for targeted and pH-responsive anticancer drug delivery. ACS Appl Mater Interfaces. 2014;6(15):11882–90. https://doi.org/10.1021/am502423r.
Song J, Wang F, Yang X, Ning B, Harp MG, Culp SH, Hu S, Huang P, Nie L, Chen J, Chen X. Gold nanoparticle coated carbon nanotube ring with enhanced Raman scattering and photothermal conversion property for theranostic applications. J Am Chem Soc. 2016;138(22):7005–15. https://doi.org/10.1021/jacs.5b13475.
Song C, Dou Y, Yuwen L, Sun Y, Dong C, Li F, Yang Y, Wang L. A gold nanoflower-based traceable drug delivery system for intracellular SERS imaging-guided targeted chemo-phototherapy. J Mater Chem B. 2018a;6(19):3030–9. https://doi.org/10.1039/c8tb00587g.
Song S, Chong Y, Fu H, Ning X, Shen H, Zhang Z. HP-β-CD functionalized Fe3O4/CNPs-based theranostic nanoplatform for pH/NIR responsive drug release and mr/nirfl imaging-guided synergetic chemo/photothermal therapy of tumor. ACS Appl Mater Interfaces. 2018b;10(40):33867–78. https://doi.org/10.1021/acsami.8b09999.
Song S, Shen H, Wang Y, Chu X, **e J, Zhou N, Shen J. Biomedical application of graphene: from drug delivery, tumor therapy, to theranostics. Colloids Surf B Biointerfaces. 2020;185:110596. https://doi.org/10.1016/j.colsurfb.2019.110596.
Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1(3):203–12. https://doi.org/10.1007/s12274-008-8021-8.
Tang X, Yu H, Bui B, Wang L, **ng C, Wang S, Chen M, Hu Z, Chen W. Nitrogen-doped fluorescence carbon dots as multi-mechanism detection for iodide and curcumin in biological and food samples. Bioact Mater. 2020;6(6):1541–54. https://doi.org/10.1016/j.bioactmat.2020.11.006.
Tao Y, Ju E, Liu Z, Dong K, Ren J, Qu X. Engineered, self-assembled near-infrared photothermal agents for combined tumor immunotherapy and chemo-photothermal therapy. Biomaterials. 2014;35(24):6646–56. https://doi.org/10.1016/j.biomaterials.2014.04.073.
Thirunavukkarasu GK, Cherukula K, Lee H, Jeong YY, Park IK, Lee JY. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials. 2018;180:240–52. https://doi.org/10.1016/j.biomaterials.2018.07.028.
Tikhonov VE, Stepnova EA, Babak VG, Yamskov IA, Palma-Guerrero J, Jansson HB. Bactericidal and antifungal activities of a low molecular weight chitosan and its n-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr Polym. 2006;64(1):66–72. https://doi.org/10.1016/j.carbpol.2005.10.021.
Ting S, Furong C, Jianqin Y, Jun C, Kui L, Yuji P. Hierarchical nanocomposites of graphene oxide and pegylated protoporphyrin as carriers to load doxorubicin hydrochloride for trimodal synergistic therapy. J Mater Chem B. 2018;6(28):4687–96. https://doi.org/10.1039/c8tb00733k.
Tran AV, Shim K, Thi TT, Kook JK, An SS, Lee SW. Targeted and controlled drug delivery by multifunctional mesoporous silica nanoparticles with internal fluorescent conjugates and external polydopamine and graphene oxide layers-sciencedirect. Acta Biomater. 2018;74:397–413. https://doi.org/10.1016/j.actbio.2018.05.022.
Trindade GS, Farias SL, Rumjanek VM, Capella MA. Methylene blue reverts multidrug resistance: sensitivity of multidrug resistant cells to this dye and its photodynamic action. Cancer Lett. 2000;151(2):161–7. https://doi.org/10.1016/s0304-3835(99)00408-5.
Vila M, Matesanz MC, Gonçalves G, Feito MJ, Linares J, Marques PA, Portolés MT, Vallet-Regi M. Triggering cell death by nanographene oxide mediated hyperthermia. Nanotechnology. 2014;25(3): 035101. https://doi.org/10.1088/0957-4484/25/3/035101.
Wang K, Ruan J, Song H, Zhang J, Wo Y, Guo S, Cui D. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6(1):8. https://doi.org/10.1007/s11671-010-9751-6.
Wang Y, Wang H, Liu D, Song S, Wang X, Zhang H. Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials. 2013;34(31):7715–24. https://doi.org/10.1016/j.biomaterials.2013.06.045.
Wang F, Sun Q, Feng B, Xu Z, Zhang J, Xu J, Lu L, Yu H, Wang M, Li Y, Zhang W. Polydopamine-functionalized graphene oxide loaded with gold nanostars and doxorubicin for combined photothermal and chemotherapy of metastatic breast cancer. Adv Healthc Mater. 2016;5(17):2227–36. https://doi.org/10.1002/adhm.201600283.
Wang C, Zhang Z, Chen B, Gu L, Li Y, Yu S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J Colloid Interface Sci. 2018;516:332–41. https://doi.org/10.1016/j.jcis.2018.01.073.
Wang X, Yu G, Zhang J, Yu M, Ramakrishna S, Long Y. Conductive polymer ultrafine fibers via electrospinning: preparation, physical properties and applications. Prog Mater Sci. 2021;115: 100704. https://doi.org/10.1016/j.pmatsci.2020.100704.
Wen LY, Bae SM, Do JH, Park KS, Ahn WS. The effects of photodynamic therapy with photodithazine on hpv 16 e6/e7 associated cervical cancer model. J Porphyrins Phthalocyanines. 2011;15(3):174–80. https://doi.org/10.1142/S1088424611003082.
Wu C, He Q, Zhu A, Li D, Xu M, Yang H, Liu Y. Synergistic anticancer activity of photo- and chemoresponsive nanoformulation based on polylysine-functionalized graphene. ACS Appl Mater Interfaces. 2014;6(23):21615–23. https://doi.org/10.1021/am5066128.
Wu SY, An SS, Hulme J. Current applications of graphene oxide in nanomedicine. Int J Nanomed. 2015;10:9–24. https://doi.org/10.2147/IJN.S88285.
Xu WP, Zhang LC, Li JP, Lu Y, Yu SH. Facile synthesis of silver@graphene oxide nanocomposites and their enhanced antibacterial properties. J Mater Chem. 2011;21(12):4593–7. https://doi.org/10.1039/C0JM03376F.
Xu W, Kattel K, Park JY, Chang Y, Kim TJ, Lee GH. Paramagnetic nanoparticle T1 and T2 MRI contrast agents. Phys Chem Chem Phys. 2012;14(37):12687–700. https://doi.org/10.1039/c2cp41357d.
Xu Z, Wang S, Li Y, Wang M, Shi P, Huang X. Covalent functionalization of graphene oxide with biocompatible poly(ethylene glycol) for delivery of paclitaxel. ACS Appl Mater Interfaces. 2014;6(19):17268–76. https://doi.org/10.1021/am505308f.
Xu H, Fan M, Elhissi AM, Zhang Z, Wan KW, Ahmed W, Phoenix DA, Sun X. PEGylated graphene oxide for tumor-targeted delivery of paclitaxel. Nanomedicine. 2015;10(8):1247–62. https://doi.org/10.2217/nnm.14.233.
Xu Y, Yu H, Chudal L, Pandey NK, Chen W. Striking luminescence phenomena of carbon dots and their applications as a double ratiometric fluorescence probes for h2s detection. Mater Today Phys. 2020;17: 100328. https://doi.org/10.1016/j.mtphys.2020.100328.
Yan J, Zhang H, Cheng F, He Y, Su T, Zhang X, Zhang M, Zhu Y, Li C, Cao J, He B. Highly stable RGD/disulfide bridge-bearing star-shaped biodegradable nanocarriers for enhancing drug-loading efficiency, rapid cellular uptake, and on-demand cargo release. Int J Nanomed. 2018;13:8247–68. https://doi.org/10.2147/IJN.S179906.
Yan J, Chen J, Zhang N, Yang Y, Zhu W, Li L, He B. Mitochondria-targeted tetrahedral DNA nanostructures for doxorubicin delivery and enhancement of apoptosis. J Mater Chem B. 2020;8(3):492–503. https://doi.org/10.1039/c9tb02266j.
Yan J, Zhang N, Zhang Z, Zhu W, Li B, Li L, Pu Y, He B. Redox-responsive polyethyleneimine/tetrahedron DNA/doxorubicin nanocomplexes for deep cell/tissue penetration to overcome multidrug resistance. J Control Release. 2021;329:36–49. https://doi.org/10.1016/j.jconrel.2020.11.050.
Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–23. https://doi.org/10.1021/nl100996u.
Yang K, Wan J, Zhang S, Zhang Y, Lee ST, Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano. 2011;5(1):516–22. https://doi.org/10.1021/nn1024303.
Yang HW, Lu YJ, Lin KJ, Hsu SC, Huang CY, She SH, Liu HL, Lin CW, **ao MC, Wey SP, Chen PY, Yen TC, Wei KC, Ma CC. EGRF conjugated PEGylated nanographene oxide for targeted chemotherapy and photothermal therapy. Biomaterials. 2013a;34(29):7204–14. https://doi.org/10.1016/j.biomaterials.2013.06.007.
Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem Soc Rev. 2013b;42(2):530–47. https://doi.org/10.1039/c2cs35342c.
Yang L, Tseng YT, Suo G, Chen L, Yu J, Chiu WJ, Huang CC, Lin CH. Photothermal therapeutic response of cancer cells to aptamer–gold nanoparticle-hybridized graphene oxide under NIR illumination. ACS Appl Mater Interfaces. 2015;7(9):5097–106. https://doi.org/10.1021/am508117e.
Yang J, Zhai S, Qin H, Yan H, **ng D, Hu X. NIR-controlled morphology transformation and pulsatile drug delivery based on multifunctional phototheranostic nanoparticles for photoacoustic imaging-guided photothermal-chemotherapy. Biomaterials. 2018;176:1–12. https://doi.org/10.1016/j.biomaterials.2018.05.033.
Yao M, Ma L, Li L, Zhang J, Lim RX, Chen W, Zhang Y. A new modality for cancer treatment—nanoparticle mediated microwave induced photodynamic therapy. J Biomed Nanotechnol. 2016;12(10):1835–51. https://doi.org/10.1166/jbn.2016.2322.
Yi L, Zhang Y, Shi X, Du X, Wang X, Yu A, Zhai G. Recent progress of functionalised graphene oxide in cancer therapy. J Drug Target. 2019;27(2):125–44. https://doi.org/10.1080/1061186X.2018.1474359.
Yu X, Gao D, Gao L, Lai J, Zhang C, Zhao Y, Zhong L, Jia B, Wang F, Chen X, Liu Z. Inhibiting metastasis and preventing tumor relapse by triggering host immunity with tumor-targeted photodynamic therapy using photosensitizer-loaded functional nanographenes. ACS Nano. 2017;11(10):10147–58. https://doi.org/10.1021/acsnano.7b04736.
Yue H, Wei W, Yue Z, Wang B, Luo N, Gao Y, Ma D, Ma G, Su Z. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials. 2012;33(16):4013–21. https://doi.org/10.1016/j.biomaterials.2012.02.021.
Zare M, Bastami M, Solali S, Alivand MR. Aberrant miRNA promoter methylation and EMT-involving miRNAs in breast cancer metastasis: diagnosis and therapeutic implications. J Cell Physiol. 2018;233(5):3729–44. https://doi.org/10.1002/jcp.26116.
Zhang M, Cao Y, Chong Y, Ma Y, Zhang H, Deng Z, Hu C, Zhang Z. Graphene oxide based theranostic platform for T1-weighted magnetic resonance imaging and drug delivery. ACS Appl Mater Interfaces. 2013;5(24):13325–32. https://doi.org/10.1021/am404292e.
Zhang D, Wu M, Zeng Y, Wu L, Wang Q, Han X, Liu X, Liu J. Chlorin e6 conjugated poly(dopamine) nanospheres as PDT/PTT dual-modal therapeutic agents for enhanced cancer therapy. ACS Appl Mater Interfaces. 2015a;7(15):8176–87. https://doi.org/10.1021/acsami.5b01027.
Zhang H, Wu H, Wang J, Yang Y, Wu D, Zhang Y, Zhang Y, Zhou Z, Yang S. Graphene oxide-BaGdF5 nanocomposites for multi-modal imaging and photothermal therapy. Biomaterials. 2015b;42:66–77. https://doi.org/10.1016/j.biomaterials.2014.11.055.
Zhang Y, Dai T, Wang M, Vecchio D, Chiang LY, Hamblin MR. Potentiation of antimicrobial photodynamic inactivation mediated by a cationic fullerene by added iodide: in vitro and in vivo studies. Nanomedicine. 2015c;10(4):603–14. https://doi.org/10.2217/nnm.14.131.
Zhang Z, Wang M, Gao D, Luo D, Liu Q, Yang J, Li Y. Targeted Raman imaging of cells using graphene oxide-based hybrids. Langmuir ACS J Surf Colloids. 2016;32(40):10253–8. https://doi.org/10.1021/acs.langmuir.6b02248.
Zhang B, Wang Y, Liu J, Zhai G. Recent developments of phototherapy based on graphene family nanomaterials. Curr Med Chem. 2017a;24(3):268–91. https://doi.org/10.2174/0929867323666161019141817.
Zhang DY, Zheng Y, Tan CP, Sun JH, Zhang W, Ji LN, Mao ZW. Graphene oxide decorated with Ru (II)-polyethylene glycol complex for lysosome-targeted imaging and photodynamic/photothermal therapy. ACS Appl Mater Interfaces. 2017b;9(8):6761–71. https://doi.org/10.1021/acsami.6b13808.
Zhang P, Wang Y, Lian J, Shen Q, Wang C, Ma B, Zhang Y, Xu T, Li J, Shao Y, Xu F, Zhu JJ. Engineering the surface of smart nanocarriers using a pH-/thermal-/GSH-responsive polymer zipper for precise tumor targeting therapy in vivo. Adv Mater. 2017c. https://doi.org/10.1002/adma.201702311.
Zhang Q, Wu Z, Li N, Pu Y, Wang B, Zhang T, Tao J. Advanced review of graphene-based nanomaterials in drug delivery systems: synthesis, modification, toxicity and application. Mater Sci Eng C Mater Biol Appl. 2017d;77:1363–75. https://doi.org/10.1016/j.msec.2017.03.196.
Zhang XJ, Cai WB, Hao LY, Hu XH, Wei XJ, Wang XY. Preparation of thermo/ph-sensitive reduced graphene oxide interpenetrating hydrogel nanocomposites for co-delivery of paclitaxel and epirubicin. Mater Technol. 2017e. https://doi.org/10.1080/10667857.2017.1410987.
Zhang C, Liu Z, Zheng Y, Geng Y, Han C, Shi Y, Sun H, Zhang C, Chen Y, Zhang L, Guo Q, Yang L, Zhou X, Kong L. Glycyrrhetinic acid functionalized graphene oxide for mitochondria targeting and cancer treatment in vivo. Small. 2018. https://doi.org/10.1002/smll.201703306.
Zhang Y, Chen S, An J, Fu H, Wu X, Pang C, Gao H. Construction of an antibacterial membrane based on dopamine and polyethylenimine cross-linked graphene oxide. ACS Biomater Sci Eng. 2019;5(6):2732–9. https://doi.org/10.1021/acsbiomaterials.9b00061.
Zhang X, Liang T, Ma Q. Layer-by-layer assembled nano-drug delivery systems for cancer treatment. Drug Deliv. 2021;28(1):655–69. https://doi.org/10.1080/10717544.2021.1905748.
Zhao L. A novel graphene oxide polymer gel platform for cardiac tissue engineering application. 3 Biotech. 2019;9(11):401. https://doi.org/10.1007/s13205-019-1912-4.
Zhao X, Wei Z, Zhao Z, Miao Y, Qiu Y, Yang W, Jia X, Liu Z, Hou H. Design and development of graphene oxide nanoparticle/chitosan hybrids showing pH-sensitive surface charge-reversible ability for efficient intracellular doxorubicin delivery. ACS Appl Mater Interfaces. 2018;10(7):6608–17. https://doi.org/10.1021/acsami.7b16910.
Zhen Z, Tang W, Chuang YJ, Todd T, Zhang W, Lin X, Niu G, Liu G, Wang L, Pan Z, Chen X, **e J. Tumor vasculature targeted photodynamic therapy for enhanced delivery of nanoparticles. ACS Nano. 2014;8(6):6004–13. https://doi.org/10.1021/nn501134q.
Zhi X, Fang H, Bao C, Shen G, Zhang J, Wang K, Guo S, Wan T, Cui D. The immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials. 2013;34(21):5254–61. https://doi.org/10.1016/j.biomaterials.2013.03.024.
Zhou T, Zhou X, **ng D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials. 2014;35(13):4185–94. https://doi.org/10.1016/j.biomaterials.2014.01.044.
Zhu S, Meng Q, Wang L, Zhang J, Song Y, ** H, Zhang K, Sun H, Wang H, Yang B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem. 2013;52(14):3953–7. https://doi.org/10.1002/anie.201300519 (International ed. in English).
Zou X, Yao M, Ma L, Hossu M, Han X, Juzenas P, Chen W. X-ray-induced nanoparticle-based photodynamic therapy of cancer. Nanomedicine. 2014;9(15):2339–51. https://doi.org/10.2217/nnm.13.198.
Zou X, Zhang L, Wang Z, Luo Y. Mechanisms of the antimicrobial activities of graphene materials. J Am Chem Soc. 2016;138(7):2064–77. https://doi.org/10.1021/jacs.5b11411.
Acknowledgements
None.
Funding
This study was supported by the National Natural Science Foundation of China [22008130], the China Postdoctoral Science Foundation (2020M682124), Qingdao Postdoctoral Researchers Applied Research Project Foundation (RZ2000001426), Scientific Research Foundation for Youth Scholars from Qingdao University (DC1900014265), the Major Science and Technology Innovation Projects of Shandong Province [2018CXGC1408], the Science and Technology Projects for people’s livelihood of Qingdao [18-6-1-93-nsh].
Author information
Authors and Affiliations
Contributions
Conception/design: LL and QM. Collection and/or assembly of data: LL, QM, YG, JC, SH, YL and TZ. Manuscript writing: LL, QM and Yang Song. Final approval of manuscript: QM, JC, Yang Song and Yong Sun. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Current study is available from the corresponding author on reasonable request.
Competing interests
All of the authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, L., Ma, Q., Cao, J. et al. Recent progress of graphene oxide-based multifunctional nanomaterials for cancer treatment. Cancer Nano 12, 18 (2021). https://doi.org/10.1186/s12645-021-00087-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12645-021-00087-7