Abstract
Background
Squamous cell carcinoma has been attributed to chronic schistosomiasis and is the predominant type of bladder cancer in schistosomiasis endemic areas. The aim of this study was to assess early promoter DNA methylation in selected genes implicated in schistosomiasis-associated bladder cancer (SABC).
Methods
A total of 159 urine samples were collected from school-aged children in Eggua Community of Ogun State and examined by microscopy for Schistosoma haematobium eggs. From this sample, a subset of 34 (21.1%) urine samples positive for S. haematobium, age and sex-matched with negative urine control samples, and 16 formalin-fixed paraffin-embedded bladder cancer tissues obtained from the University College Hospital were subjected to DNA isolation and bisulphite DNA conversion. Quantitative methylation-specific PCR was used to determine the methylation status of APC, RARβ2, RASSF1A, and TIMP3 in the samples.
Results
High degrees of methylation of RARβ2(67.7%), RASSF1A (38.2%), and TIMP3(52.9%) was more common in urogenital schistosomiasis (UGS)-positive urine samples than negative urine (control) samples and in bladder cancer tissues. Promoter DNA methylation in the positive urine samples was 1.4-fold, 13.3-fold, 3.4-fold, and 3.8-fold higher in APC, RARβ2, RASSF1A, and TIMP3, respectively, than in the matched controls. The odds of promoter methylation were likely to increase with age group for APC (OR: 1.615) and TIMP3(OR: 2.000); sex for TIMP3(OR: 2.644); and haematuria for RARβ2(OR: 1.094), RASSF1A (OR: 1.143), and TIMP3(OR: 1.842), although there were no significant associations. Conclusions: Gene promoter DNA methylation in tumour suppressor genes was observed in schistosomiasis cases. Hence, promoter DNA methylation may occur during active schistosomiasis in children. This result may serve as an early non-invasive biomarker to detect and hint at the risk of develo** SABC later in life.
Similar content being viewed by others
1 Background
1.1 Schistosomiasis
Schistosomiasis is a neglected tropical disease (NTD) endemic in sub-Saharan Africa, the Middle East and Asia [50]. It is a water-borne disease caused by species of the genus Schistosoma. The endemic species to Africa are S. haematobium and S. mansoni which cause urogenital schistosomiasis and intestinal schistosomiasis, respectively. Individuals get infected through contact with water bodies contaminated with the parasite infective larval stage (cercaria). Data from the World Health Organization [49] show that schistosomiasis is present in 78 countries, and it is estimated that at least 90% of people requiring treatment for schistosomiasis reside in Africa. It has been estimated that over 700 million people worldwide in endemic regions are at risk of infection, with over 200,000 deaths annually [46].
The host immune response to the various stages of the life cycle of Schistosoma haematobium is inflammatory. The consequence of the inflammatory response is granulomatous formation around eggs lodged in the tissues of the bladder. The granulomata usually conjugate, forming tubercles and nodules that often ulcerate [5]. In chronic schistosomiasis, this leads to several morbidities such as anaemia, undernutrition, dysuria and female genital sores [3] and may result in malignant transformation (squamous cell carcinoma), which usually presents at a late stage [16].
In addition, lesions due to schistosomiasis are assumed to play a part in intensifying the exposure of the bladder epithelium to mutagenic substrates from tobacco or other known toxic chemicals. Although the mechanisms underlying the link between schistosomiasis and bladder cancer are still debatable, available data show that schistosomiasis induces DNA methylation via chronic inflammation [4, 8, 39]. DNA methylation is known to be involved in numerous cancer phenotypes [24, 34] and has been the research focus primarily for diagnostic, prognostic, and therapeutic purposes.
1.2 Schistosomiasis-induced DNA Methylation and Bladder Cancer
DNA methylation is an epigenetic mechanism in the mammalian genome involving transferring a methyl group onto the C5 position of cytosine to form 5-methylcytosine. It regulates gene expression by recruiting proteins involved in gene repression or inhibiting transcription factor binding [31]. During development, the DNA methylation pattern changes due to de novo and demethylation processes, resulting in differentiated cells develo** a stable and unique pattern that regulates tissue-specific gene transcription [31]. Naturally, DNA methylation is carried out by a group of DNA methyltransferase enzymes (DNMTs), namely DNMT3a and DNMT3b and DNMT1 [6]. The former group is responsible for de novo DNA methylation during embryonic development, while DNMT1 maintains the methylation in subsequent cell division using hemimethylated strands [29]. This allows for normal gene regulation and expression.
In contrast, DNA methylation patterns can be altered directly or indirectly by disease causing pathogens (bacteria, parasites, and viruses) [15] or chemical agents like amines, tobacco and arsenic [7]. This has debilitating effects on gene expression and function, thus, impacting disease progression and resistance to therapy. Aberrant DNA methylation (hyper- and hypomethylation) has been associated with numerous diseases including cancer. Many cancerous cells are characterized by different abnormal DNA methylation patterns, which are usually distinct and can be used to discriminate between cancerous and normal cells [16].
Chronic schistosomiasis, which synchronizes with chronic inflammation, peaks in late adolescence [19]. It has also been established that chronic inflammation elicits aberrant DNA methylation [32], which is believed to constitute the initiation phase in cancer development [28]. Thus, DNA methylation may constitute some of the early events leading up to Schistosomiasis Associated Bladder Cancer (SABC) and may occur in children with active schistosomiasis infection. Therefore, assessing DNA methylation patterns in infected children may aid in identifying individuals who may be at risk of develo** SABC later in life and provide the basis for early intervention.
Schistosomiasis Associated Bladder Cancer (SABC) has not been documented in Eggua, southwest Nigeria, but Onile et al. [36] reported that about 62% of bladder pathologies detected by ultrasonography were associated with chronic schistosomiasis. This may suggest that altered DNA methylation patterns would have occurred. In addition, there are reports of cancer-specific DNA methylation alterations in pre-diagnostic blood collected more than 10 years before diagnosis of chronic lymphocytic leukaemia [27]. Based on these reports and the fact that children are more susceptible to Schistosoma haematobium infection, it can be suggested that epigenetic changes begin early in children during active infections. Thus, they are hotspots for events leading up to disease progression, bladder pathologies and risk of develo** schistosomiasis-associated bladder cancer later in life.
1.3 DNA Methylation as biomarker for SABC
The gold standard for the diagnosis, and sometimes treatment, of bladder cancer is cystoscopy, which is invasive and painful. DNA methylation biomarkers have been widely used for early diagnosis, prognosis and prediction of diseases, including cancer. These biomarkers are easily (non-invasively and non-painfully) obtainable from body fluids. Different DNA methylation biomarkers have been assessed for detection, prognosis and treatment of SABC which usually presents late [16]. It is known that the best form of treatment is prevention and that DNA methylation events (reversible) can occur years before diagnosis of neoplasm, hence, assessing these events at their earliest onset is pertinent. Therefore, the use of non-invasive DNA methylation biomarkers to identify early events in childhood schistosomiasis may help to speed up intervention measures and treatments.
Four genes, APC, RARβ2, RASSF1A, and TIMP3, were selected from the literature for this study. These genes are widely used in biomarker studies for bladder cancer, including schistosomiasis-associated bladder cancer (SABC). APC is a tumour suppressor gene that regulates the Wnt signalling pathway and is frequently mutated in SABC cases. Higher methylation rates of APC have been identified as a potential urine biomarker for the early detection of SABC [16]. RARB2 is a retinoic acid receptor gene involved in cell differentiation and proliferation and is frequently hypermethylated in SABC cases. RASSF1A is a tumour suppressor gene that regulates cell cycle progression and apoptosis and is frequently hypermethylated in SABC cases. TIMP3 is a tissue inhibitor of the metalloproteinases gene involved in extracellular matrix remodelling and is frequently hypermethylated in SABC cases [51]. These genes are associated with tumour progression, recurrence, and differential methylation patterns and have been used as biomarkers for SABC diagnosis, prognosis, and early detection [13, 50]. Detection of methylation in these genes offers valuable information for early detection and treatment.
This study is the first attempt to evaluate DNA methylation induced by S. haematobium infection in children. We also attempt to establish a possible link between S. haematobium infection, especially in children, and SABC. This study will contribute to understanding epigenetic changes of schistosomiasis in childhood. It will also contribute to the understanding of how these changes predispose infected individuals to develo** SABC. Evaluation of the methylation status will determine which urine biomarkers can be used and are effective in hinting at the risk of develo** SABC.
This study is aimed at evaluating promoter DNA methylation as an early non-invasive potential biomarker in children infected with Schistosoma haematobium in Eggua. We have established that DNA methylation is induced during childhood schistosomiasis, especially in tumour suppressor genes. Therefore, untreated schistosomiasis infection can leave the individual at risk of develo** schistosomiasis-associated bladder cancer later in life.
2 Materials and methods
2.1 Study design
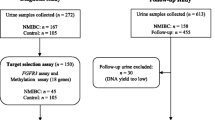
For this study, a cross-sectional design was used to collect urine samples once from voluntary participants who were all school-aged children. A total of 159 participants between the ages of 5 and 16 years were recruited from Eggua Community, using informed consent procedures. A total of 16 formalin-fixed paraffin-embedded (FFPE) bladder cancer tissue blocks (SABC and Non-SABC) were collected from the cancer registry of the University College Hospital (UCH), University of Ibadan (UI). The cancer tissue blocks were analysed by a Histopathologist from the University College Hospital (UCH), University of Ibadan, to ascertain if they were bladder cancer tissues and their histological type.
2.2 Ethical approval
Ethical approval was obtained from the UI/UCH Health Research Ethics Committee, College of Medicine, University of Ibadan (IRB number: UI/UCH/22/0036). Using informed consent procedures, the assent and consent of the children and their parents/guardians were obtained in writing before sampling was conducted.
2.3 Study area
Eggua is a rural agricultural community. The primary water source is the River Yewa, which is used for domestic purposes, including drinking, washing and cooking, fishing, and swimming. Schistosomiasis is known to be prevalent in this community [1], and bladder pathologies associated with Schistosomiasis have also been reported [36].
2.4 Study population
The study population was drawn from school-aged children living in the study area. Urine samples from participants positive for Schistosoma eggs and the cancer tissue blocks were used as cases, while samples negative for parasite eggs or haematuria served as controls.
2.5 Inclusion criteria
Children under 18 years of age were recruited in this study. Only urine samples positive for the parasite were used for further DNA methylation analysis. Children under 18 years were recruited because they have a naïve immune system and are most susceptible to the parasite insult. Moreover, after adolescence, there are less apparent events that may indicate active infection, giving rise to a “concomitant immunity” with a balanced host–parasite inter-relationship. This keeps both alive and establishes reduced parasite fertility, limited patient morbidity, and resistance to re-infection [5].
2.6 Sample size determination
This was determined according to Charan and Biswas [11] and calculated using the formula below.
Values for proportions were obtained based on the number of cases and control from a previous study [2]. Thus, number of cases = 57, number of control = 13, r = 0.23, expected proportion in cases (p1) = 0.81, and expected proportion in control (p2) = 0.19, Zβ = 1.28, Zβ/2 = 1.96, p* = 0.5.
Therefore, samples from a minimum of 37 participants were used in this study.
2.7 Biological sample collection and analysis
Voided urine (20 ml) was collected from each participant in reagent bottles. Urinalysis reagent strips (Rapid Labs, UK) were used to rapidly detect blood and analytes, including glucose, ketone, specific gravity, pH, proteinuria, and leukocytes in the urine samples, following the manufacturer’s instructions. Samples were immediately centrifuged at 3000 g for 10 min, and the sediment carefully examined by microscopy for presence of Schistosoma eggs. The number of parasite eggs was counted and recorded as eggs/10 mL of urine. The intensity of infection was categorized as light (< 50 eggs/10 mL of urine) or heavy (≥ 50 eggs/10 mL of urine), as previously described by WHO expert committee [47].
Urine samples were then stored at − 30 °C until DNA extraction was carried out. Tissue blocks of formalin-fixed paraffin-embedded specimens of Schistosoma-associated bladder cancer and urothelial bladder cancer were obtained from the bladder cancer registry of UCH, University of Ibadan. Genomic DNA was extracted from them.
2.8 Gene selection for promoter methylation
Using information from the literature, genes were chosen based on their specific methylation state for infection, inflammation, and cancer. Four genes (APC, RARβ2, RASSF1A, and TIMP3) associated with schistosomiasis-induced bladder pathology and cancers [16, 51] were selected for analysis of methylation abnormalities. These genes have been shown to be highly sensitive as biomarkers for bladder cancer. RASSF1A, APC and RARβ2 are all tumour suppressor genes, while TIMP3 plays a crucial role in activating apoptotic cascade.
2.9 Primer design for APC, RARβ2, RASSF1A, and TIMP3
The gene ID and organism of the target genes were obtained from the National Centre for Biotechnology Information (NCBI) database. This was then used to search for the promoter region of the gene in the Eukaryotic Promoter Database (EPD). The sequence of the promoter region for each target gene was then retrieved from the database using the sequence retrieval tool. A BLAST search for the promoter sequence was carried out using the NCBI BLAST tool to determine specificity of the promoter sequence. Primer design specific for methylation assay was then carried out using MethPrimer as previously reported [41].
2.10 Genomic DNA extraction from bladder cancer tissues and urine samples
For genomic DNA extraction, four tissue sections (≤ 20 µm thick) were obtained from each bladder cancer tissue block, using a microtome and transferred to 1.5-ml microcentrifuge tubes for deparaffinization.
2.10.1 Deparaffinization of tissue sections and DNA extraction
This was carried out using the Zymo Research Quick-DNA FFPE kit, following the manufacturer’s instructions. Briefly, 400 μL of deparaffinization solution was added to 1.5-ml microcentrifuge tubes, incubated at 55 °C for 1 min, and thereafter vortexed briefly. The deparaffinazation solution was then discarded. Deparaffinized tissue was digested using Proteinase K, dH2O, and 2X digestion buffer. This was incubated at 55 °C overnight for 12–16 h. DNA was purified and eluted with 70μL of DNA Elution Buffer. Purified DNA was then stored at ≤ − 20 °C for further use.
2.10.2 DNA isolation from urine samples
Thirty-four urine samples positive for Schistosoma eggs were used for DNA extraction. DNA was also extracted from 34 urine samples that were microscopically negative for Schistosoma eggs and this served as a control. DNA isolation was done using the Geneaid gDNA Microkit, following the manufacturer’s instructions. Urine (1 ml) was transferred to a 1.5-ml microcentrifuge tube and centrifuged at 6000xg for 2 min and the supernatant discarded. This was resuspended using 500 μL of elution buffer, centrifuged at 600xg for 1 min, and the supernatant discarded. Cells were lysed with 200 μL of S1 buffer and 20 μL of Proteinase K and incubated at 60 °C for 30 min. Further lysis was done with 200 μL of S2 buffer and incubated at 60 °C for 20 min. DNA was bound using 200 μL of absolute ethanol, washed and eluted with 60 μL of DNA elution buffer. Eluted DNA was stored at ≤ − 20 °C for further use. The total amount of DNA yield per sample ranged from 500 ng to 2 μg.
2.11 Bisulfite treatment of isolated DNA
DNA extracted from urine samples and paraffinized tissue was subjected to bisulphite treatment, which converts unmethylated cytosine residues to uracil residues, leaving the methylated cytosines as previously reported [25]. Briefly, 500 ng to 2 μg of genomic DNA from each sample were denatured using 2 μl of freshly prepared NaOH (final concentration, 3 M) and incubated at 37 °C for 10 min. The denatured DNA was then mixed with freshly prepared sodium bisulphite solution (final concentration, 5 M), covered with mineral oil and incubated in the dark at 50 °C for 16 h. The heavy mineral oil was carefully separated from the reaction solution and the bisulfite-modified DNA were purified using GenepHlow Gel/PCR purification kit, following manufacturer’s instructions. Modified DNA was then stored at − 80 °C.
2.12 Quantitative methylation-specific PCR (qMSP) for bisulfite-converted DNA
The qMSP were conducted for each bisulfite-modified DNA on Applied Systems Bio-Rad iQ5 Thermocycler. Amplification was carried out in a 25 µl total reaction volume containing 4 µl of master mix (5 × HOT FIREPol EvaGreen qPCR Supermix), 0.4 μl each of forward and reverse primers for each gene (APC, RARβ2, RASSF1A, and TIMP3), 18.2 µl of nuclease free water, and 2 μl of modified DNA. Amplification reaction conditions were as follows: hot start at 95 °C for 5 min, subsequent denaturation at 95 °C for 15 min, annealing at 48–49 °C for 15 min, extension at 72 °C for 20 min, and final extension step at 55 °C for 10 min. This was carried out for 40 cycles. Amplification was carried out for methylated and unmethylated primers of each gene separately. Melt curves were also generated for each reaction. No template control (NTC) was used as the negative control. The level of CpG island methylation for promoter region of each gene was obtained among three categories of samples, i.e. infection only (urogenital schistosomiasis alone), bladder cancer tissues, and controls (no infection/urogenital schistosomiasis). Primer sequences, their parameters, and qMSP conditions are shown in Additional file 1: Table S1.
2.13 Interpretation and data analysis for qMSP
The average melt temperature was used to ascertain actual amplified product for each primer. Relative quantification normalized against unit mass (number of samples) was used to determine fold change with the control sample as the calibrator. This was determined using; Ratio(test/calibrator) = 2ΔCt, where ΔCt = Ct(calibrator)–Ct(test).
2.14 Statistical analysis
All statistical analysis was done using SPSS version 20. Logistic regression analysis was used to test association between promoter region methylation of each gene with age group, sex, intensity of infection, and haematuria. Odds ratio was used to predict the risk of promoter methylation with age group, sex, intensity of infection, and haematuria.
3 Results
A total of 159 school-aged children participated in this study. Out of this, 76(47.8%) were males while 83 (52.2%) were females, with a mean age of 10.6 years (Table 1). A total of 34 urine samples were positive for Schistosoma haematobium (Sh) eggs after microscopic examination for a prevalence of 21.4%. Of this, light infection (< 50 eggs/10 mL of urine) occurred in 32(94.1%) samples, while heavy infection (≥ 50 eggs/10 mL of urine) was seen in 2(5.9%) samples. Urinalysis showed that 33(20.8%) of the participants had haematuria, out of which 11(33.3%) were positive for S. haematobium infection (Table 1). An equal number (17) of males (22.4%) and females (20.5%) had S. haematobium infection (Table 1).
A total of 68 urine samples were then subjected to further analysis of DNA methylation; 34 were positive for urogenital schistosomiasis (UGS), and urine samples from 34 age and sex-matched participants without S. haematobium eggs and haematuria and, thus, served as controls.
A total of 16 archived FFPE bladder cancer tissues were composed of 12(75%) males, while females were 4(25%) with an average age of 55.8 years. More males (75%) than females (25%) had bladder cancer (Table 1). Urothelial carcinoma was the most common type of tumour, constituting 50% of the samples (Table 1).
3.1 Gene promoter methylation of the target genes in schistosomiasis
Promoter methylation in the following genes, APC, RARβ2, RASSF1A, and TIMP3, was evaluated. RARβ2 was the most methylated in 23(67.7%) of all the samples, while APC was the least methylated in 9(26.5%) samples (Table 2). Compared to the controls, RARβ2 was methylated in 8(23.5%), while RASSF1A was the least methylated in 2(5.9%) of the control samples.
Promoter DNA methylation in the positive urine samples was 1.4-fold, 13.3-fold, 3.4-fold, and 3.8-fold higher in APC, RARβ2, RASSF1A, and TIMP3, respectively, than in the matched controls. The same genes were analysed in established tumour tissues to ascertain whether the epigenetic alterations in UGS urine sediments were identical to those of established tumours. The percentage methylation of the genes in tumour tissue and UGS urine sediment DNA which were similar are shown in Fig. 1.
In comparing urine samples and bladder cancer tissue, high degrees of methylation of RARβ2, RASSF1A, and TIMP3 were more common in UGS-positive urine samples than negative (control) samples (Table 2) and more common in bladder cancer tissues as well (Fig. 1). Additionally, while a high degree of methylation occurred for RARβ2 and was closely followed by TIMP3 for UGS-positive urine samples, the reverse was the case for bladder cancer tissues (Fig. 1).
3.2 Statistical analysis
Logistic regression analysis was used to test the association between the promoter methylation of the genes of interest and age group, sex, intensity of infection, and haematuria for urogenital schistosomiasis (Table 3).
As shown in Table 3, the majority of the participants with urogenital schistosomiasis belong to the age group 11–16 years, and this group also accounted for the highest number of promoter methylation of the tested genes. However, there was no association between age and promoter methylation (p > 0.05).
As shown in Table 1, equal numbers of male and female children were infected with Schistosoma haematobium. While males had the highest promoter methylation for RARβ2, RASSF1A and TIMP3, females only had the highest promoter hypermethylation in APC (Table 3). There was no association between sex and promoter methylation in children with schistosomiasis (p > 0.05).
Most promoter methylation occurred in light infection intensity, as this study’s overall intensity was low. There was no association between the intensity of infection and promoter methylation, p > 0.05 (Table 3). A high degree of promoter DNA methylation for all the tested genes occurred in participants whose urine had no haematuria. There was no association between haematuria and promoter DNA methylation p > 0.05 (Table 3).
The odds ratio was used to predict if age group, sex, intensity of infection and haematuria were associated with gene promoter DNA methylation. Our results showed that promoter DNA methylation of APC and TIMP3 were associated with age groups, with children ages 6–10 having higher odds of promoter methylation, but there was no significant association (Table 4). Sex was associated with promoter DNA methylation of TIMP3, with males more likely to have promoter methylation, but it was not significant (Table 4). Interestingly, haematuria was associated with promoter methylation of 3 genes; RARβ2, RASSF1A, and TIMP3, although there was no significant association (Table 4).
4 Discussion
Altered DNA methylation patterns can inform, (and have been used) in the diagnosis, prognosis, treatment, and management of different cancers and other diseases and disorders [9]. Schistosoma haematobium, the causative agent of schistosomiasis, a neglected tropical disease, is a designated Class I carcinogen implicated in SABC [28]. Although its mechanism of action is still debatable, it is believed that S. haematobium induces bladder cancer over time via chronic inflammation [28, 32]. Schistosomiasis-associated bladder cancer usually presents late and has poor prognosis.
We propose that assessing DNA methylation patterns elicited by chronic inflammation during active chronic infection in children could provide the basis for early intervention. In the present study, the data showed a high degree of methylation in the promoter region of four genes (APC, RARβ2, RASFF1A, and TIMP3) which are more common in UGS-positive urine sediments of children, than negative UGS urine controls, and more common in bladder cancer tissues as well. These may suggest that events leading up to SABC begin during active infection in children. This observation can be used to distinguish between cases and non-cases and identify individuals at risk of develo** SABC later in life.
4.1 Epidemiology of schistosomiasis in Eggua
The overall prevalence of urogenital schistosomiasis in this study was 21.4%. This is lower than 30.8% reported for children in the same area by Oladele et al. [35]. Meanwhile, Alexander et al. [3] reported a lower prevalence of 14% in children in other areas of Ogun State. The low prevalence of urogenital schistosomiasis recorded in this study may result from the ongoing mass drug administration in Ogun State. It is reported that at least once a year since 2017, pupils in the state are treated with praziquantel depending on the availability of the drugs [3].
Opara et al. [37] found that males and females were equally infected with the parasite. This corroborates the observations in the present study, with equal number (17) of males (22.4%) and females (20.5%) infected with S. haematobium. However, this is at variance with other reports that observed high infection in males than females [46] and in females than in males [3]. This has been attributed to the study areas’ socio-cultural practices and how frequently individuals contact cercariae-infested water bodies.
Intensity of infection is usually associated with chronicity of disease, clinical manifestations, morbidities, and bladder pathologies. Most of those who were schistosomiasis-positive had only light infection, as has consistently been seen in this area [36] and other parts of the country [3, 37]. Conversely, reports from other parts of Nigeria [19] and Ghana [43] found that the majority of participants had a heavy infection (≥ 50 eggs/10 mL urine). Only two participants had heavy infections in the current study, similar to the report of Alexander et al. [3].
4.2 Hypermethylation of gene promoter region in childhood UGS
In the current study, the promoter region RARβ2, RASSF1A, and TIMP3 showed a high degree of promoter methylation in 23(67.7%), 13(38.2%), and 18(52.9%), respectively, in UGS-positive urine than in negative UGS urine control samples (Table 2). Similarly, we compared this methylation pattern in urine with that of bladder cancer tissues and found a high degree of promoter methylation of the target genes (Fig. 1). The observations of the current study show that schistosomiasis in children may induce DNA methylation in the target genes due to chronic inflammation. Egg deposition in tissues usually corresponds with inflammatory response [12]. With the observation of eggs in urine of infected children in this study, it can be suggested that chronic inflammation was taking place and may be the reason for the high degrees of methylation of promoter regions of the target genes.
To ascertain that the target genes are markers for bladder cancer, we tested for promoter region DNA methylation of the target genes in bladder cancer tissues. Our observations showed marked promoter DNA methylation in the target genes and support our observation of the same promoter DNA methylation in childhood schistosomiasis. This is similar to the report of Zaghloul et al. [50], which found DNA methylation of promoter regions of these genes and suggested their implications for bladder cancer. This implies that these genes are markers for bladder cancer. The reports that cancer-specific DNA methylation can occur more than ten years before diagnosis of neoplasm [27] also support the observation of DNA methylation in children with schistosomiasis in the current study. They may imply that events leading up to SABC at a later stage in life may begin years before diagnosis. This is in addition to the fact that altered DNA methylation pattern is associated with the initiation phase of cancer or precedes tumorigenesis [22].
4.3 DNA methylation in childhood schistosomiasis
In the current study, it was observed that the promoter regions of APC, RARβ2, RASSF1A and TIMP3 were 1.4 times, 13.3 times, 3.4 times, and 3.8 times more methylated, respectively, in schistosomiasis-positive urine samples than in the negative urine controls. The presence of DNA methylation in non-tumour urine cells in this study, validated by those in the invasive bladder cancer tissues, may suggest the presence of an “epigenetic field defect”.
Kim and Kim, [22] found that normal-appearing tissues taken at least 5 cm away from invasive tumours had 169 hypermethylated loci; 142 loci were the same as those seen in the invasive tumours. This they described as an “epigenetic field defect”, meaning that methylation was already present before initiation of tumorigenesis. Furthermore, the study’s findings indicate that S. haematobium can effectively induce DNA methylation in non-cancerous exfoliated cells. This is similar to the report of Nakajima et al. [33] that Helicobacter pylori can effectively induce aberrant DNA methylation in non-cancerous gastric mucosae.
The “field of cancerization” concept was first reported when it was observed that metachronous primary cancers developed further, even after curative resection in oral cavity cancer [42]. The presence of “epigenetic field defect” may contribute to what is referred to as an “epigenetic field of cancerization”. This implies that aberrant DNA methylation in non-tumour cells may suggest the role of the former in the epigenetic field of cancerization [33]. Therefore, the observations of promoter hypermethylation of tumour suppressor genes in the present study may hint that during schistosomiasis in children, DNA methylation alterations occur, forming an epigenetic field of cancerization which may, over time, progress to SABC.
The observations of the current study show that the highest number of promoter methylation of the tested genes occurred in children with schistosomiasis in the age group 11–16 years old (Fig. 1; Table 3). This may be because schistosomiasis infection peaks at adolescence [19] and synchronizes with time when chronic inflammation, restorative hyperplasia, squamous cell metaplasia, and DNA lesions may be high and may later evolve into bladder cancer [43]. Although there was no association between age and promoter region hypermethylation, these results suggest that DNA methylation events and pre-cancerous lesions may begin during active schistosomiasis infection in childhood.
In the present study, there was no association between sex and promoter DNA methylation for the tested genes in schistosomiasis-positive samples. This may imply that DNA methylation of the promoter region of the tested genes was independent of sex. This may explain why more males than females have bladder cancer [21].
The intensity of infection in this study was low and may explain why there is no association with promoter DNA hypermethylation of APC, RARβ2, RASSF1A, and TIMP3. In contrast, a study reports an association between light intensity and adult bladder pathologies [36]. Therefore, even in light infections, chronic disease can still occur, leading to epigenetic alterations like DNA methylation, which may be more easily observed than mutations and precede cytological abnormalities, morbidities, bladder pathologies, and the risk of malignant transformation later in life.
The odds ratio was used to predict the risk of gene promoter DNA methylation with the age groups, sex, intensity of infection, and haematuria. Interestingly, haematuria was associated with promoter methylation of three genes: RARβ2, RASSF1A, and TIMP3, although it was not statistically significant. Haematuria is a classical sign of ongoing urogenital schistosomiasis [10, 48], which may explain the high degrees of promoter DNA methylation of the tested genes in this study. Based on these observations, it can be said that once infection with S. haematobium is established, gene promoter DNA methylation will occur. Therefore, it could be suggested that since DNA methylation occurs before or marks the onset of future malignant transformation, childhood schistosomiasis marks the beginning of events leading up to SABC.
4.4 Possible link between childhood UGS and the risk of develo** SABC at a later stage in life
It is known that children under 18 years are the most susceptible group to schistosomiasis infection [20], which may result from children having a naïve immune system [40]. Infection is highest in children from 5 years old and peaks in adolescents of age between 15 and 18 years [46]. This may explain why there are usually less apparent events that may indicate active infection after adolescence, giving rise to a “concomitant immunity” with a balanced host–parasite inter-relationship. This keeps both alive and establishes a reduced parasite’s fertility, limited patient morbidity, and resistance to re-infection infection [5]. Furthermore, it has been established that DNA methylation occurred in pre-diagnostic blood collected more than 10 years before diagnosis of chronic lymphocytic leukaemia [27]. Moreover, the role of epigenetic alterations appears to not only be limited to cancers, and there is even a greater possibility that “epigenetic field defect” may play a part in and be identified for various diseases [33].
Based on the preceding observations and the presence of an “epigenetic field defect” in the current study, it can be suggested that DNA methylation begins during active schistosomiasis infection in children under the age of 18 years old. According to Nakajima et al. [33], measuring disease risk at a time point using a DNA methylation marker will aid individuals change their lifestyles, especially for close intensive disease prevention. The results of the current study suggest that the epigenetic alterations occurring during childhood schistosomiasis may be the link to SABC at a later stage in life.
These observations imply that early events of DNA methylation of promoter region of tumour suppressor genes (APC, RARβ2, and RASSF1A) and TIMP3, involved in apoptosis may occur during active infections of S. haematobium in childhood. These alterations, if not repaired or incompletely repaired, may be replicated and passed on from one generation of cells to another. This may lead to the transformation of cells , as seen in urothelial hyperplasia and squamous cell metaplasia in children with active schistosomiasis infection [43]. Urothelial hyperplasia and squamous metaplasia are themselves potential preneoplastic lesions of the bladder [17]. Subsequently, these lesions may promote the propagation of cells harbouring genotoxic DNA damage and, in a matter of time, further genotoxic damage before potential cancer occurs [45].
Thus, hypermethylated genes during childhood infections may be maintained and carried from one generation of cells to another until infection peaks in late adolescence. At this point, granulomas may have accumulated to be replaced by restorative hyperplasia. This may explain why children with UGS may have such mild bladder pathologies as thickness of bladder wall and irregularities [30]. These are silent events that are almost unobservable and may serve as the link between chronic UGS in childhood and SABC in later age.
The occurrence of high degrees of promoter DNA methylation of tumour suppressor genes as seen in the present study is not conclusive that cancer will develop. Indeed, if the disease is left untreated in childhood, there is a higher risk of cancer development later. It is evident that epigenetic changes are reversible [38]; therefore, DNA methylation induced by schistosomiasis may be reversed if detected early, thereby preventing progression or development of the cancer. Tetteh-Quarcoo et al. [44] reported post-praziquantel treatment reversal of cytological abnormalities such as transitional metaplastic squamous cells to normal cells in children after 3–8 weeks. Drugs such as DNA methylase inhibitors or histone deacetylase inhibitors are known to restore the activity of suppressed genes.
Therefore, early detection and drugs targeted at reversal of epigenetic changes are areas of research interest. More studies are needed to validate the observations from the present study, especially to check if there is an association between DNA methylation and bladder pathology not just in children with active infection, but also in adults who may have had schistosomiasis as children or presenting with morbidity markers. Furthermore, this study is limited by the small size. Hence, there is a need for a large sample size or cohort studies. Additionally, further studies can be done with individuals with a history of urogenital schistosomiasis to establish a possible link between childhood urogenital schistosomiasis and SABC later in life.
This is important because S. haematobium may leave behind fingerprints of DNA damage like DNA methylation which may induce carcinogenesis later in life. Some carcinogenic factors are known to leave their fingerprint such as specific DNA methylation patterns in the tissues they damage even if they are no longer present or eradicated, for example H. pylori [26]. Moreover, DiNardo et al. [14] found that schistosomiasis-induced CD4+ T cells DNA methylation signature persisted at least 6 months after successful deworming for schistosomiasis. This indicates the continued effect of schistosome infections even after treatment and is consistent with observations that the pathologies associated with schistosomiasis persists beyond infection [23].
Although it is a schistosomiasis endemic area, it is noteworthy that SABC has not been reported in Eggua community. Although this claim has not been fully verified, there might be few explanations. First, as infected individuals grow, they acquire effective immunity to the parasite insult, which may aid in adequate moderation of immune response to the infection. Malignant transformation is usually a result of poorly regulated infection, which may lead to fibrosis and SABC [4]. Secondly, during childhood, abnormal cells may have resolved either by themselves or these individuals may have had treatment for the infection. This can be explained by the fact that transitional squamous metaplastic cells are known to resolve with time in children, especially post-praziquantel treatment Tetteh-Quarcoo et al. [44].
Finally, schistosomiasis usually works together with other factors like environmental carcinogens and mutagens to induce bladder cancer [18]. Therefore, these individuals may not be at risk of persistent exposure to these genotoxins at levels high enough to elicit malignant transformation. Nevertheless, further studies are needed to verify these assertions.
5 Conclusion
The present study has shown that gene promoter region DNA methylation of APC, RARβ2, RASSF1A, and TIMP3 will occur once Schistosoma haematobium infection is established especially in the most vulnerable group (children). The observation of an “epigenetic field of cancerization” in this study could be used as a molecular biomarker to identify individuals at risk of malignant transformation in later age. Based on the presence of an “epigenetic field of cancerization”, it can be suggested that the journey to malignant transformation begins during active infection in childhood, especially if disease is left untreated. Therefore, individuals, especially children living in schistosomiasis endemic areas, should be given adequate public health attention through adequate diagnosis, mass drug administration, routine follow-up, and means of avoiding contact with infested water bodies.
Availability of data and materials
All the data is available in the paper.
Abbreviations
- BC:
-
Bladder cancer
- DNMT:
-
DNA Methyltransferase
- EPD:
-
Eukaryotic Promoter Database
- FFPE:
-
Formalin-fixed paraffin-embedded
- NCBI:
-
National Centre for Biotechnology Information
- NTC:
-
No Template Control
- NTD:
-
Neglected Tropical Disease
- PCR:
-
Polymerase Chain Reaction
- qMSP:
-
Quantitative Methylation-Specific PCR
- SABC:
-
Schistosomiasis-associated bladder cancer
- UCH:
-
University College Hospital
- UGS:
-
Urogenital schistosomiasis
- UI:
-
University of Ibadan
- WHO:
-
World Health Organization
References
Adebayo AS, Mundhe SD, Awobode HO, Onile OS, Agunloye AM et al (2018) Metabolite profiling for biomarkers in Schistosoma haematobium infection and associated bladder pathologies. PLoS Negl Trop Dis 12:4
Adebayo AS, Survayanshi M, Bhute S, Agunloye AM, Isokpehi RD, Anumudu CI et al (2017) The microbiome in urogenital schistosomiasis and induced bladder pathologies. PLoS Negl Trop Dis 11(8):e0005826
Alexander SI, Nwafor TS, Gyang PV, Idowu ET, Akinwale OP (2021) Prevalence of urogenital schistosomiasis and its implication on control efforts among school pupils in Ogun State. Southwest Nigeria PAJOLS 5(3):335–341
Arora N, Kaur R, Anjum F, Tripathi S, Mishra A, Kumar R, Prasad A (2019) Neglected agent eminent disease: linking human helminthic infection, inflammation, and malignancy. Fron Cell Infect Microbio 9:402
Barsoum RS, Esmat G, El-Baz T (2013) Human schistosomiasis: clinical perspective: review. J Adv Res 4:433–444
Bestor TH (2000) The DNA methyltransferases of mammals. Human Molec Genet 9:2395–2402
Bjørklund G, Aaseth J, Chirumbolo S, Urbina MA, Uddin R (2018) Effects of arsenic toxicity beyond epigenetic modifications. Environ Geochem Health 40(3):955–965
Botelho MC, Alves H, Richter J (2017) Halting schistosoma haematobium—associated bladder cancer. Int J Cancer Manag 109(9):e9430
Cao J, Wu Q, Huang Y, Wang L, Su Z, Ye H (2021) The role of DNA methylation in syndromic and non-syndromic congenital heart disease. Clin Epigenetics 13(1):93
Center for Disease Control, CDC (2020) Schistosomiasis. https://www.cdc.gov/parasites/schistosomiasis/health_professionals/index.html. Accessed 30 July 2023
Charan J, Biswas T (2013) How to calculate sample size for different study designs in medical research? Indian J Psychol Med 35(2):121–126
Colley DG, Secor WE (2014) Immunology of human schistosomiasis. Parasite Immunol 36(8):347–357
Conti SL, Honeycutt J, Odegaard JI, Gonzalgo ML, Hsieh MH (2015) Alterations in DNA methylation may be the key to early detection and treatment of schistosomal bladder cancer. PLoS Negl Trop Dis 9(6):e0003696
DiNardo AR, Nishiguchi T, Mace EM, Rajapakshe K, Mtetwa G, Kay A et al (2018) Schistosomiasis induces persistent DNA methylation and tuberculosis-specific immune changes. J Immunol 201(1):124–133
Djomkam Zune AL, Olwal CO, Tapela K, Owoicho O, Nganyewo NN, Lyko F et al (2021) Pathogen-induced epigenetic modifications in cancers: implications for prevention, detection and treatment of cancers in Africa. Cancers 13:6051
Eissa S, Swellam M, El-Khouly IM, Kassim SK, Shehata H, Mansour A, Esmat M, Nossier AI, Hamdy MA, Awad NM, El-Ahmady O (2011) Aberrant methylation of RARbeta2 and APC genes in voided urine as molecular markers for early detection of bilharzial and nonbilharzial bladder cancer. Canc Epidemiol Biomark Prev: Publ Am Assoc Canc Res, Cospons Am Soci Prevent Oncol 20(8):1657–1664
Honeycutt J, Hammam O, Fu C, Hsieh MH (2014) Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol 30(7):324–332
Ishida K, Hsieh MH (2018) Understanding urogenital schistosomiasis-related bladder cancer: an update. Front Med 5:223
Jiya FB, Jiya NM, Ibitoye PK, Mohammed Y, Mohammad AU (2022) Chronic urogenital schistosomiasis among in-school adolescents in Sokoto. North-Western Nigeria AJMAH 20(1):22–36
Kaleson MS, Istifanus WA, Suleiman MM, Panda SM (2022) Urogenital schistosomiasis among communities surrounding Kiri Reservoir, Adamawa State. Nigeria AJHSE 3(1):01–10
Kibaru J, Kotecha P, Iya AM et al (2021) Sco** review protocol: bladder cancer in Nigeria: what are the gaps in clinical care and research? BMJ Open 11:e041894
Kim YJ, Kim WJ (2016) Can we use methylation markers as diagnostic and prognostic indicators for bladder cancer? Investig Clin Urol 57(Suppl 1):S77-88
King CH (2015) It’s time to dispel the myth of “asymptomatic” schistosomiasis. PLoS Negl Trop Dis 9:e0003504
Lakshminarasimhan R, Liang G (2016) The role of DNA methylation in cancer. Adv Exp Med Biol 945:151–172
Li Y, Tollefsbol TO (2011) DNA methylation detection: bisulfite genomic sequencing analysis. Methods Mol Biol 791:11–21. https://doi.org/10.1007/978-1-61779-316-5_2
Liu, D., Liu, Y., Zhu, W. et al (2023) Helicobacter pylori-induced aberrant demethylation and expression of GNB4 promotes gastric carcinogenesis via the Hippo–YAP1 pathway. BMC Med 21:134. https://doi.org/10.1186/s12916-023-02842-6
Loi E, Moi L, Fadda A, Satta G, Zucca M, Sanna S et al (2019) Methylation alteration of SHANK1 as a predictive, diagnostic and prognostic biomarker for chronic lymphocytic leukemia. Oncotarget 10(48):4987–5002
Lori H, Egan LJ (2012) Inflammation, DNA methylation and colitis-associated cancer. Carcinogenesis 33(4):723–731
Martisova A, Holcakova J, Izadi N, Sebuyoya R, Hrstka R, Bartosik M (2021) DNA methylation in solid tumors: functions and methods of detection. Int J Mol Sci 22(8):4247
Mawa PA, Kincaid-Smith J, Tukahebwa EM, Webster JP, Wilson S (2021) Schistosomiasis morbidity hotspots: roles of the human host, the parasite and their interface in the development of severe morbidity. Front Immunol 12:635869
Moore L, Le T, Fan G (2013) DNA methylation and its basic function. Neuropsychopharmacol 38:23–38
Murata M (2018) Inflammation and cancer. Environ Health Prev Med 23(1):50
Nakajima T, Enomoto S, Ushijima T (2018) DNA methylation: a marker for carcinogen exposure and cancer risk. Environ Health Prev Med 13:8–15
Nishiyama A, Nakanishi M (2021) Navigating the DNA methylation landscape of cancer. TIG 37(11):1012–1027
Oladele VS, Awobode H, Anumudu CI (2014) Subtle morbidities associated with malaria co-infection with schistosomiasis among children in South-West Nigeria. Afr J Med Med Sci 43(Suppl):125–135
Onile OS, Awobode HO, Oladele VS, Agunloye AM, Anumudu CI (2016) Detection of urinary tract pathology in some Schistosoma haematobium infected Nigerian Adults. J Trop Med 2016:5405207
Opara KN, Akomalafe RT, Udoidung NI, Afia UU, Yaro CA, Bassey BE (2021) Urogenital schistosomiasis among primary school children in rural communities in Obudu. Southern Nigeria Int J MCH AIDS 10(1):70–80
Porten SP (2018) Epigenetic alterations in bladder cancer. Curr Urol Rep 19:102
Santos LL, Santos J, Gouveia MJ, Bernardo C, Lopes C, Rinaldi G, Costa BPJ, Costa JM (2021) Urogenital schistosomiasis—history, pathogenesis, and bladder cancer. J Clin Med 10:205
Sekar K, Krishnamurthy S, Mandal J, Rajappa M (2023) Global DNA methylation in children with complicated urinary tract infection. Arch Intern Med Res 3:001–009
Sigalotti L, Covre A, Colizzi F, Fratta E (2019) Quantitative methylation-specific PCR: a simple method for studying epigenetic modifications of cell-free DNA. Methods Mol Biol 1909:137–162
Slaughter DP, Southwick HW, Smejkal W (1953) Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6:963–968
Tetteh-Quarcoo PB, Akuetteh BK, Owusu IA, Quayson SE, Attah SK, Armah R et al (2019) Cytological and wet mount microscopic observations made in urine of schistosoma haematobium-infected children: hint of the implication in bladder cancer. Can J Infect Dis Med Microbiol. https://doi.org/10.1155/2019/7912186
Tetteh-Quarcoo PB, Ampong A, Dayie NTKD, Ahenkorah J, Udofia EA, Afutu E et al (2022) Dynamics in morbidity markers and cytological observations made in urine of schistosoma haematobium-infected children: pre and post-praziquantel treatment in an endemic setting. Med Sci 10:14
Vennervald BJ (2015) Epidemiology and mechanism of carcinogenesis of schistosomiasis. Trop Hem-Onc. https://doi.org/10.1007/978-3-319-18257-5-18
Verjee MA (2019) Schistosomiasis: still a cause of significant morbidity and mortality. Res Rep Trop Med 10:153–163
WHO Expert Committee (2002) Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ. Tech. Rep. Ser. 912
World Health Organization, WHO (2023) Schistosomiasis. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed 30 July 2023
World Health Organization, WHO (2022) https://www.who.int/newsroom/factsheets/detail/schistosomiasis. Accessed 30 July 2023
Zaghloul MS, Zaghloul TM, Bishr MK, Baumann BC (2020) Urinary schistosomiasis and the associated bladder cancer: update. J Egypt Nat Cancer Inst 32:44
Zhong X, Isharwal S, Naples JM, Shiff C, Veltri RW, Shao C, Bosompem KM, Sidransky D, Hoque MO (2013) Hypermethylation of genes detected in urine from Ghanaian adults with bladder pathology associated with Schistosoma haematobium infection. PLoS ONE 8(3):e59089. https://doi.org/10.1371/journal.pone.0059089
Acknowledgements
CIA acknowledges ARNTD SGP IV Grant 2020. We appreciate the members of staff of African Biosciences for their support during the quantitative methylation-specific PCR.
Funding
This publication was supported by a Grant No. (SGPIV/0121.102) from the African Research Network for Neglected Tropical Diseases (ARNTD) through United States Agency for International Development (USAID), UK aid from the British people, and the Coalition for Operational Research on Neglected Tropical Diseases (COR-NTD).
Author information
Authors and Affiliations
Contributions
CAA and CIA contributed to conception or design. CAA, OEF, RPE, HOA, and CIA performed data acquisition. CAA, OEF, and CIA performed analysis or interpretation. CAA, OEF, RPE, HOA, and CIA performed critical review. CAA, OEF, RPE, HOA, and CIA made final version approval.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the UI/UCH Health Research Ethics Committee, College of Medicine, University of Ibadan (IRB No.: UI/UCH/22/0036). Using informed consent procedures, the assent and consent of the children and their parents/guardians were obtained in writing before sampling was conducted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1: Primer Sequences and qMSP reaction conditions.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Akpabio, C.A., Ebuh, R.P., Fatunla, O.E. et al. DNA methylation profiles in urothelial bladder cancer tissues and children with schistosomiasis from Eggua, Ogun State, Nigeria. Afr J Urol 29, 65 (2023). https://doi.org/10.1186/s12301-023-00392-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12301-023-00392-0