Abstract
Background
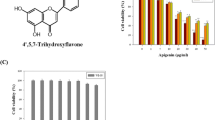
Apigenin (4’,5,7-trihydroxyflavone) was recently shown effective in inhibiting several cancers. The aim of this study was to investigate the effect and mechanism of apigenin in the human bladder cancer cell line T24 for the first time.
Methods
T24 cells were treated with varying concentrations and time of apigenin. Cell viability was evaluated by MTT assay. Cell motility and invasiveness were assayed by Matrigel migration and invasion assay. Flow cytometry and western blot analysis were used to detect cell apoptosis, cell cycle and signaling pathway.
Results
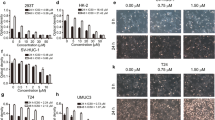
The results demonstrated that apigenin suppressed proliferation and inhibited the migration and invasion potential of T24 bladder cancer cells in a dose- and time-dependent manner, which was associated with induced G2/M Phase cell cycle arrest and apoptosis. The mechanism of action is like to involve PI3K/Akt pathway and Bcl-2 family proteins. Apigenin increased caspase-3 activity and PARP cleavage, indicating that apigenin induced apoptosis in a caspase-dependent way.
Conclusions
These findings suggest that apigenin may be an effective way for treating human bladder cancer.
Similar content being viewed by others
Background
Apigenin, one of the most common flavonoids, is widely distributed in many fruits and vegetables, including parsley, onions, orange, tea, chamomile, wheat sprouts and in some seasonings [1]. Apigenin has potential uses in cancer prevention and therapy, and it suppresses cell growth against many human cancer cell lines, including breast, colon, skin, thyroid, leukemia, and prostate cancer cells [2–5]. Unlike other structurally related flavonoids, apigenin is non-mutagenic [6]. Although previous reports have shown the inhibitory effect of apigenin on other human cancer cells, there are few reports indicating the inhibitory effect on human bladder cancer cells.
Bladder cancer is the most common malignant tumor of the urinary tract. Worldwide, bladder cancer is the seventh most common cancer. An average of 386,300 new cases of urinary bladder cancer are diagnosed worldwide every year, accounting for 150,200 deaths [7]. In recent decades, bladder cancer was shown of a rising overall incidence [8]. In most cases of nonmuscle invasive bladder cancer, tumors are treated initially with TURBT (Transurethral Resection of Bladder Tumor). A careful cystoscopic examination of the entire urethra and all bladder surfaces precedes resection. Intravesical therapy can also be employed in an expectant way as opposed to an induction course alone to provide long-term immunostimulation or chemotoxicity and thereby prevent disease recurrence [9, 10]. Our earlier studies have shown that EGCG [Cell apoptosis assay The extent of apoptosis was evaluated by annexin V-FITC and flow cytometry. Cells were grown at a density of 1 × 106 cells in six-well culture dishes and were treated with different concentrations of apigenin (0–80 μM in DMSO) for 24 h. Following treatment, the cells were harvested, washed twice with pre-chilled PBS, and resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. One hundred microliters of such solution was mixed with 5 μL annexin V-FITC and 5 μL propidium iodide for 15 min, and then 400 μL 1× binding buffer was added. Analysis was carried out using a FC500 flow cytometer with CXP software (Beckman Coulter, Fullerton, CA, USA) within 1 h. The percentage of apoptotic cells was assessed by CXP software. Cells were plated in six-well culture dishes at concentrations determined to yield 60–70% confluence within 24 h. Cells were then treated with different concentrations of apigenin (0–160 μM in DMSO). After 24 h, cells were washed twice with PBS then centrifuged. The pellet was fixed with 70% ethanol for 1 h at 4°C. The cells were washed with PBS and resuspended with propidium iodide solution (0.05 mg/mL) containing RNase, incubated at room temperature in the dark for 30 min. DNA content was then analyzed using the FC500 flow cytometer. Cell were harvested at 24 h following apigenin treatment, washed, and lysed with lysis buffer (10 mmol/L Tris-HCl, 0.25 mol/ L sucrose, 5 mmol/L EDTA, 50 mmol/L NaCl, 30 mmol/L sodium pyrophosphate, 50 mmol/L NaF, 1 mmol/LNa3VO4, 1 mmol/L PMSF, and 2% cocktail [pH 7.5]). Protein concentration in the resulting lysate was determined using the bicinchoninic acid protein assay. Appropriate amounts of protein (20–30 μg) were separated by electrophoresis in 10–12% Tris-glycine polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked then incubated overnight with the appropriate primary antibody at dilutions specified by the manufacturer. They were next washed and incubated with the corresponding HRP-conjugated secondary antibody at 1:1000 dilution in Tris-buffered saline-Tween 20 (10 mM Tris-Cl [pH 7.4], 150 mM NaCl, 0.1% Tween-20). Bound secondary antibody was detected using an enhanced chemiluminescence system (Pierce Biotechnology). Statistical significance was compared between various treatment groups and controls using ANOVA. Data were considered statistically significant when P-values were <0.05.Cell cycle assay
Western blot analysis
Statistical analysis
References
Birt DF, Mitchell D, Gold B, Pour P, Pinch HC: Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res. 1997, 17 (1A): 85-91.
Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M: Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int J Cancer. 2000, 87 (4): 595-600. 10.1002/1097-0215(20000815)87:4<595::AID-IJC21>3.0.CO;2-5.
Wang IK, Lin-Shiau SY, Lin JK: Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer. 1999, 35 (10): 1517-1525. 10.1016/S0959-8049(99)00168-9.
Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF: Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000, 28 (2): 102-110. 10.1002/1098-2744(200006)28:2<102::AID-MC6>3.0.CO;2-2.
Yin F, Giuliano AE, Law RE, Van Herle AJ: Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001, 21 (1A): 413-420.
Czeczot H, Tudek B, Kusztelak J, Szymczyk T, Dobrowolska B, Glinkowska G, Malinowski J, Strzelecka H: Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutat Res. 1990, 240 (3): 209-216. 10.1016/0165-1218(90)90060-F.
Jemal ABF, Center MM, Ferlay J, Ward E, Forman D: Global cancer statistics. CA Cancer J Clin. 2011, 61: 69-90. 10.3322/caac.20107.
Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK: Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003, 95 (17): 1276-1299. 10.1093/jnci/djg040.
De Boer EC, De Jong WH, Steerenberg PA, Aarden LA, Tetteroo E, De Groot ER, Van der Meijden AP, Vegt PD, Debruyne FM, Ruitenberg EJ: Induction of urinary interleukin-1 (IL-1), IL-2, IL-6, and tumour necrosis factor during intravesical immunotherapy with bacillus Calmette-Guerin in superficial bladder cancer. Cancer Immunol Immunother. 1992, 34 (5): 306-312. 10.1007/BF01741551.
Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, Sarosdy MF, Bohl RD, Grossman HB, Beck TM: Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000, 163 (4): 1124-1129. 10.1016/S0022-5347(05)67707-5.
Qin J, **e LP, Zheng XY, Wang YB, Bai Y, Shen HF, Li LC, Dahiya R: A component of green tea, (-)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res Commun. 2007, 354 (4): 852-857. 10.1016/j.bbrc.2007.01.003.
Bai Y, Mao QQ, Qin J, Zheng XY, Wang YB, Yang K, Shen HF, **e LP: Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010, 101 (2): 488-493. 10.1111/j.1349-7006.2009.01415.x.
Boatright KM, Salvesen GS: Mechanisms of caspase activation. Curr Opin Cell Biol. 2003, 15 (6): 725-731. 10.1016/j.ceb.2003.10.009.
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C: PI3K/Akt and apoptosis: size matters. Oncogene. 2003, 22 (56): 8983-8998. 10.1038/sj.onc.1207115.
Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE: Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000, 275 (15): 10761-10766. 10.1074/jbc.275.15.10761.
Vivanco I, Sawyers CL: The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002, 2 (7): 489-501. 10.1038/nrc839.
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME: Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997, 91 (2): 231-241. 10.1016/S0092-8674(00)80405-5.
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC: Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998, 282 (5392): 1318-1321.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME: Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999, 96 (6): 857-868. 10.1016/S0092-8674(00)80595-4.
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB: NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999, 401 (6748): 82-85. 10.1038/43466.
Romashkova JA, Makarov SS: NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999, 401 (6748): 86-90. 10.1038/43474.
Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN: Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci U S A. 1997, 94 (8): 3627-3632. 10.1073/pnas.94.8.3627.
Medema RH, Kops GJ, Bos JL, Burgering BM: AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000, 404 (6779): 782-787. 10.1038/35008115.
Masuelli L, Marzocchella L, Quaranta A, Palumbo C, Pompa G, Izzi V, Canini A, Modesti A, Galvano F, Bei R: Apigenin induces apoptosis and impairs head and neck carcinomas EGFR/ErbB2 signaling. Front Biosci. 2011, 16: 1060-1068. 10.2741/3735.
Asnaghi L, Calastretti A, Bevilacqua A, D'Agnano I, Gatti G, Canti G, Delia D, Capaccioli S, Nicolin A: Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene. 2004, 23 (34): 5781-5791. 10.1038/sj.onc.1207698.
Chan TO, Rittenhouse SE, Tsichlis PN: AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999, 68: 965-1014. 10.1146/annurev.biochem.68.1.965.
Brown GC, Borutaite V: Nitric oxide, cytochrome c and mitochondria. Biochem Soc Symp. 1999, 66: 17-25.
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X: Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997, 275 (5303): 1129-1132. 10.1126/science.275.5303.1129.
King KL, Cidlowski JA: Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998, 60: 601-617. 10.1146/annurev.physiol.60.1.601.
Pan J, She M, Xu ZX, Sun L, Yeung SC: Farnesyltransferase inhibitors induce DNA damage via reactive oxygen species in human cancer cells. Cancer Res. 2005, 65 (9): 3671-3681. 10.1158/0008-5472.CAN-04-2744.
Lepley DM, Li B, Birt DF, Pelling JC: The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis. 1996, 17 (11): 2367-2375. 10.1093/carcin/17.11.2367.
Lepley DM, Pelling JC: Induction of p21/WAF1 and G1 cell-cycle arrest by the chemopreventive agent apigenin. Mol Carcinog. 1997, 19 (2): 74-82. 10.1002/(SICI)1098-2744(199707)19:2<74::AID-MC2>3.0.CO;2-L.
Lee SRPJ, Park EK, Chung CH, Kang SS, Bang OS: Akt-induced promotion of cell-cycle progression at G2/M phase involves upregulation of NF-Y binding activity in PC12 cells. J Cell Physiol. 2005, 205 (2): 270-277. 10.1002/jcp.20395.
Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN: A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987, 47 (12): 3239-3245.
Acknowledgements
We acknowledge all members of the Key Lab of Multi-Organ Transplantation of Health Ministry for their support. This work was supported by the National Key Clinical Specialty Construction Project of China, Key medical disciplines of Zhejiang province, Combination of traditional Chinese, Medicine and health development plan project of Zhejiang province (2012RCA016) and Western medicine key disciplines of Zhejiang Province (2012-XK-A23) and Medical Scientific Research Foundation of Zhejiang Province, China (2013KYB100).