Abstract
Background
Orchids are one of the most diversified angiosperms, but few genomic resources are available for these non-model plants. In addition to the ecological significance, Phalaenopsis has been considered as an economically important floriculture industry worldwide. We aimed to use massively parallel 454 pyrosequencing for a global characterization of the Phalaenopsis transcriptome.
Results
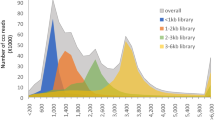
To maximize sequence diversity, we pooled RNA from 10 samples of different tissues, various developmental stages, and biotic- or abiotic-stressed plants. We obtained 206,960 expressed sequence tags (ESTs) with an average read length of 228 bp. These reads were assembled into 8,233 contigs and 34,630 singletons. The unigenes were searched against the NCBI non-redundant (NR) protein database. Based on sequence similarity with known proteins, these analyses identified 22,234 different genes (E-value cutoff, e-7). Assembled sequences were annotated with Gene Ontology, Gene Family and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Among these annotations, over 780 unigenes encoding putative transcription factors were identified.
Conclusion
Pyrosequencing was effective in identifying a large set of unigenes from Phalaenopsis. The informative EST dataset we developed constitutes a much-needed resource for discovery of genes involved in various biological processes in Phalaenopsis and other orchid species. These transcribed sequences will narrow the gap between study of model organisms with many genomic resources and species that are important for ecological and evolutionary studies.
Similar content being viewed by others
Background
The family of Orchidaceae is the largest family of flowering plants and the number of species may exceed 25,000 [1]. Like all other living organisms, present-day orchids have evolved from ancestral forms as a result of selection pressure and adaptation. They show a wide diversity of epiphytic and terrestrial growth forms and have successfully colonized almost every habitat on earth. Factors promoting orchid species richness include specific interaction between the orchid flower and pollinator [2], sequential and rapid interplay between drift and natural selection [3], obligate interaction with mycorrhiza [4], and epiphytism which is true for most of all orchids and probably two-thirds of the epiphytic flora of the world.
The radiation of the orchid family has probably taken place in a comparatively short period as compared with that of most flowering plant families, which had already started to diversify in the Mid-Cretaceous [5]. The time of origin of orchids is in dispute, although Dressler suggests that they originated 80 to 40 million years ago (Mya; late Cretaceous to late Eocene) [6]. Recently, the origin of the Orchidaceae was dated with a fossil orchid and its pollinator. The authors showed that the most recent common ancestor of extant orchids lived in the late Cretaceous (76-84 Mya) [7]. They also suggested that Epidendroideae and Orchidoideae, two of the largest orchid subfamilies, which together represent > 95% of living orchid species, began to diversify early in the Tertiary (65 Mya) [7].
According to molecular phylogenetic studies, Orchidaceae comprise 5 subfamilies: Apostasioideae, Cypripedioideae, Vanilloideae, Orchidoideae and Epidendroideae. The Apostasioideae is considered the sister group to other orchids. Vanilloideae diverged just before Cypripedioideae. Both subfamilies have relatively low numbers of genera and species. Most of the taxonomic diversity in orchids is in 2 recently expanded sister-subfamilies: Orchidoideae and especially Epidendroideae [8, 9]. Orchids are known for their diversity of specialized reproductive and ecological strategies. For successful reproduction, the production of labellum and gynostemium (a fused structure of androecium and gynoecium) to facilitate pollination is well documented and the co-evolution of orchid flowers and pollinators is well known [10, 11]. In addition, the especially successful evolutionary progress of orchids may be explained by mature pollen grains packaged as pollinia, pollination-regulated ovary/ovule development, synchronized timing of micro- and mega-gametogenesis for effective fertilization, and the release of thousands or millions of immature embryos (seeds without endosperm) in a mature capsule [12]. However, despite their unique developmental reproductive biology, as well as specialized pollination and ecological strategies, orchids remain under-represented in molecular studies relative to other species-rich plant families [13]. The reasons may be associated with the large genome size, long life cycle, and inefficient transformation system of orchids.
The genomic sequence resources currently available for orchids are limited. Very recently, a sketch of the Phalaenopsis orchid genome from sequencing the ends of 2 bacterial artificial chromosome libraries of P. equestris was reported [14]. In addition, a number of studies have developed expressed sequence tags (ESTs) resources for orchids by using Sanger sequencing [15–18]. Fewer than 12,000 ESTs, including 5,593 from P. equestris, 2,359 from P. bellina, 1,080 from Oncidium Gower Ramsey, and 2,132 from Vanda Mimi Palmer, have been deposited in public database. These studies have highlighted the utility of cDNA sequencing for discovering candidate genes for orchid floral development [19, 20], floral scent production [16, 21] or flowering time [22] in the absence of a genomic sequence. However, a comprehensive description of the full complement of gene expressed in orchids remains unavailable.
Massively parallel 454 pyrosequencing has become feasible for increasing sequencing depth and coverage while reducing time, labour, and cost [23, 24]. This technology can be used to deeply explore the nature and complexity of a given transcriptional universe. 454 sequencing of transcriptomes for model organisms has confirmed that the relatively short reads produced by this technology can be effectively assembled and used for gene discovery [54], shoot apical meristem maintenance [55], drought tolerance [56], and response to abscisic acid in Craterostigma plantagineum[57]. The AP2/ERF superfamily is defined by the AP2/ERF domain, of about 60 to 70 amino acids, and is involved in DNA binding. A combination of genetic and molecular approaches has been used to characterize a series of regulatory genes of the AP2/ERF family. The members of this family are involved in regulating various biological processes related to growth and development, as well as various responses to environmental stimuli. This family includes genes related to drought [58], high salt concentration [58], low temperature [59], diseases [60, 61], and the control of ovule development and flower organ growth [62]. Understanding the functions of these genes will advance our understanding of the great morphological diversity and successful adaptation of orchids. However, we did not find the transcription factor families LFY, M-type, STAT, VOZ, and WOX, in the Phalaenopsis transcriptome. These families might either be rarely expressed, or might not have appeared in our cDNA sampling.
Conclusion
Thanks to recent advances in next-generation sequencing technology, we have applied RNA-seq to facilitate transcriptome analysis of orchids which present important biological questions but lack a fully sequenced genome. Our findings represent substantial contributions to the publicly accessible expressed sequences for the Orchidaceae family. With the whole genome sequencing of P. equestris in progress, this collection of ESTs is a valuable resource that will be immediately useful for researchers, allowing for correction of assemblies, annotation, and construction of gene models to establish accurate exon-intron boundaries. Application of these resources through the common language of nucleotide sequences will greatly enhance the insights into the reproductive success of orchids.
methods
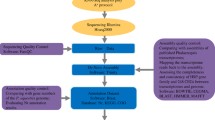
Plant materials and cDNA library construction
Phalaenopsis equestris, P. aphrodite subsp. formosana and P. bellina were grown without fungal symbiosis in greenhouses at National Cheng Kung University under natural light and controlled temperature ranging from 23°C to 27°C. To maximize the diversity and effectively collect sequences from expressed genes of orchids, we collected 10 samples from different tissues, developmental stages and treatments (Table 1). Inflorescences, flower buds, leaves and roots were sampled from the 3-year-old P. equestris. Young leaves were collected as they emerged. Old leaves were taken at the fourth leaf counting down from the newly emerged one. The cold-stressed leaves were collected from old leaves of 3-year-old plants treated for 4 hrs at 4°C. Because Erwinia chrysanthemi is one of the most serious pathogens infecting Phalaenopsis, old leaves were infected with E. chrysanthemi to induce the expression of pathogen-related genes. Protocorms were 20-day-old germinating seeds of P. aphrodite subsp. formosana grown on tissue-cultured plates without fungal symbiosis. Cool night-induced spikes were sampled from 3-year-old P. aphrodite subsp. formosana treated with cool night temperature (28°C day/20°C night) for 2 weeks to induce spike emergence [63]. P. bellina flowers with a strong fragrance were collected on day 5 post-anthesis [16]. Collected samples were frozen immediately in liquid nitrogen and stored at -80°C until used.
Total RNA from each sample was extracted separately following the method described by [19]. Poly-A RNA was prepared from each total RNA sample using the Oligotex@ mRNA Mini kit (Qiagen, Ontario, Canada). Samples of 0.5 μg mRNA from each sample were combined into a single large pool and mixed well. This single large, equally-mixed pool was the source for the cDNA library construction. The cDNA library was constructed using the SMART cDNA synthesis Kit (BD Clontech, Mountain View, CA) according to the manufacturer's instructions.
Pyrosequencing and assembly
In preparation for 454 sequencing, 5 μg of the cDNA sample was nebulized to a mean fragment size of 600 ± 50 bp, end repaired and adapter ligated according to previously published literature [23]. After streptavidin bead enrichment and DNA denaturation, single-stranded molecules were titrated onto derivatized Sepharose beads and then amplified by emulsion PCR. A second streptavidin bead enrichment followed emulsion breaking, the bead-attached DNAs were then denatured with NaOH, and sequencing primers were annealed. One 454 pyrosequencing run was carried out with use of a GS FLX sequencer. A 454 SFF file containing raw sequences and sequence quality information can be access through the SRA web site under accession number SRA030758.2.
Low quality data (base call score < 10) were trimmed from the ends of individual sequences. Sequences shorter than 50 bp after processing were excluded from the analysis. For assembly, GS FLX gsAssembler was used with minimum 40 bases overlap with at least 95% identity.
Sequence analysis and GO classification
All sequences were queried for their similarity to known sequences by use of a BLASTX algorithm [64] against the "nr" protein database. Sequence similarity was considered significant at E-value < 10-7 and the "best hits" annotation was used to represent proteins similar to those encoded by the contigs and singletons. The BLAST score (bits) used the BLOSUM 62 matrix and Existence 11, Extension 1 Gap costs for BLASTX. The GO Slim Classification for Plants, developed at TAIR (http://www.arabidopsis.org/help/helppages/go_slim_help.jsp) was used to characterize the ESTs functionally. The GO identifier of the best hit (with a cutoff of 1e-7) was attributed to the sequence. This step allowed putative functions to be assigned on the basis of the classification proposed by GO.
Characterization of ESTs by Arabidopsis Gene Family and KEGG Pathways
The TAIR9 A. thaliana annotated protein databases (ftp://ftp.arabidopsis.org/home/tair/Genes/TAIR9_genome_release/TAIR9_sequences) was downloaded. The protein sequence set was BLAST against Phalaenopsis contigs and singletons with use of the TBLASTN programs. Sequence similarity was considered significant at an E-value < 10-7. Unique sequences with BLAST matches were mapped to TAIR Gene Families and KEGG Pathways of Arabidopsis for further analysis. The TAIR Gene Family information contains 8,693 genes in 176 gene families updated on September 26, 2009. The KEGG Pathways for Arabidopsis contains 6,756 genes in 121 pathways released on May 11, 2010.
Identification of putative transcription factor-related ESTs
The protein sequences of predicted transcription factors for rice were downloaded from the Plant Transcription Factor Database (PTFDB; http://planttfdb.cbi.pku.edu.cn/). PTFDB contains information on 2,424 rice (Oryza sativa subsp. japonica) transcription factors in 56 families. For identification of transcription factor-related ESTs from Phalaenopsis, the protein sequence set of each predicted rice transcription factor family was BLAST against Phalaenopsis contigs and singletons with use of the TBLASTN programs. Sequence similarity was considered significant at E-value < 10-7.
References
Atwood JT: The size of Orchidaceae and the systematic distribution of epiphytic orchids. Selbyana. 1986, 9: 171-186.
Cozzolino S, Widmer A: Orchid diversity: an evolutionary consequence of deception?. Trends Ecol Evol. 2005, 20: 487-494. 10.1016/j.tree.2005.06.004.
Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN: Variation in sexual reproduction in orchids and its evolutionary consequence: a spasmodic journey to diversification. Biol J Linn Soc. 2005, 84: 1-54.
Otero JT, Flanagan NS: Orchid diversity - beyond deception. Trends Ecol Evol. 2006, 21: 64-65. 10.1016/j.tree.2005.11.016.
Crane PR, Friis EM, Pedersen KR: The origin and early diversification of angiosperms. Nature. 1995, 374: 27-33. 10.1038/374027a0.
Dressler RL: The orchids: Natural history and classification. 1981, Cambridge, Massachusetts, USA: Harvard University Press
Ramirez SR, Gravendeel B, Singer RB, Marshall CR, Pierce NE: Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. Nature. 2007, 448: 1042-1045. 10.1038/nature06039.
Rudall PJ, Bateman RM: Roles of synorganisation, zygomorphy and heterotopy in floral evolution: the gynostemium and labellum of orchids and other lilioid monocots. Biol Rev. 2002, 56: 784-795.
Górniaka M, Paunb O, Chase MW: Phylogenetic relationships within Orchidaceae based on a low-copy nuclear coding gene, Xdh: Congruence with organellar and nuclear ribosomal DNA results. Mol Phylogenet Evol. 2010, 56: 784-795. 10.1016/j.ympev.2010.03.003.
Yu H, Goh CJ: Molecular Genetics of Reproductive Biology in Orchids. Plant Physiol. 2001, 127: 1390-1393. 10.1104/pp.010676.
Schiestl FP, Peakall R, Mant JG, Ibarra F, Schulz C, Franke S, Francke W: The chemistry of sexual deception in an orchid-wasp pollination system. Science. 2003, 302: 437-438. 10.1126/science.1087835.
Tsai WC, Hsiao YY, Pan ZJ, Kuoh CS, Chen WH, Chen HH: The role of ethylene in orchid ovule development. Plant Sci. 2008, 175: 98-105. 10.1016/j.plantsci.2008.02.011.
Peakall R: Speciation in the Orchidaceae: confronting the challenges. Mol Ecol. 2007, 16: 2834-2837. 10.1111/j.1365-294X.2007.03311.x.
Hsu C-C, Chung Y-L, Chen T-C, Lee Y-L, Kuo Y-T, Tsai W-C, Hsiao Y-Y, Chen Y-W, Wu W-L, Chen H-H: An overview of the Phalaenopsis orchid genome through BAC end sequence analysis. BMC Plant Biol. 2011, 11: 3-10.1186/1471-2229-11-3.
Tsai WC, Hsiao YY, Lee SH, Tung CW, Wang DP, Wang HC, Chen WH, Chen HH: Expression analysis of the ESTs derived from the flower buds of Phalaenopsis equestris. Plant Sci. 2006, 170: 426-432. 10.1016/j.plantsci.2005.08.029.
Hsiao YY, Tsai WC, Kuoh CS, Huang TH, Wang HC, Wu TS, Leu YL, Chen WH, Chen HH: Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce the monoterpene biosynthesis pathway. BMC Plant Biol. 2006, 6: 14-10.1186/1471-2229-6-14.
Tan J, Wang HL, Yeh KW: Analysis of organ-specific, expressed genes in Oncidium orchid by subtractive expressed sequence tags library. Biotechnol Lett. 2005, 27: 1517-1528. 10.1007/s10529-005-1468-8.
Teh SL, Chan WS, Abdullah JO, Namasivayam P: Development of expressed sequence tag resources for Vanda Mimi Palmer and data mining for EST-SSR. Mol Biol Rep. 2010
Tsai WC, Chuang MH, Kuoh CS, Chen WH, Chen HH: Four DEF-like MADS box genes displayed distinct floral morphogenetic roles in Phalaenopsis orchid. Plant Cell Physiol. 2004, 45: 831-844. 10.1093/pcp/pch095.
Tsai WC, Lee PF, Chen HI, Hsiao YY, Wei WJ, Pan ZJ, Chuang MH, Kuoh CS, Chen WH, Chen HH: PeMADS6, a GLOBOSA/PISTILLATA-like gene in Phalaenopsis equestris involved in petaloid formation, and correlated with flower longevity and ovary development. Plant Cell Physiol. 2005, 46: 1125-1139. 10.1093/pcp/pci125.
Hsiao YY, Jeng MF, Tsai WC, Chung YC, Li CY, Wu TS, Kuoh CS, Chen WH, Chen HH: A novel homodimeric geranyl diphosphate synthase from the orchid Phalaenopsis bellina lacking a DD(X)2-4D motif. Plant J. 2008, 55: 719-733. 10.1111/j.1365-313X.2008.03547.x.
Wang CY, Chiou CY, Wang HL, Krishnamurthy R, Venkatagiri S, Tan J, Yeh KW: Carbohydrate mobilization and gene regulatory profile in the pseudobulb of Oncidium orchid during the flowering process. Planta. 2008, 227: 1063-1077. 10.1007/s00425-007-0681-1.
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, et al: Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005, 437: 376-380.
Delseny M, Han B, Hsing YI: High throughput DNA sequencing: The new sequencing revolution. Plant Sci. 2010, 179: 407-422. 10.1016/j.plantsci.2010.07.019.
Cheung F, Haas BJ, Goldberg SM, May GD, **ao Y, Town CD: Sequencing Medicago truncatula expressed sequenced tags using 454 Life Sciences technology. BMC Genomics. 2006, 7: 272-10.1186/1471-2164-7-272.
Emrich SJ, Barbazuk WB, Li L, Schnable PS: Gene discovery and annotation using LCM-454 transcriptome sequencing. Genome Res. 2007, 17: 69-73.
Varshney RK, Nayak SN, May GD, Jackson SA: Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol. 2009, 27: 522-530. 10.1016/j.tibtech.2009.05.006.
Jones-Rhoades MW, Borevitz JO, Preuss D: Genome-wide expression profiling of the Arabidopsis female gametophyte identifies families of small, secreted proteins. PLoS Genet. 2007, 3: 1848-1861.
Weber AP, Weber KL, Carr K, Wilkerson C, Ohlrogge JB: Sampling the Arabidopsis transcriptome with massive parallel pyrosequencing. Plant Physiol. 2007, 144: 32-42. 10.1104/pp.107.096677.
Ohtsu K, Smith MB, Emrich SJ, Borsuk LA, Zhou R, Chen T, Zhang X, Timmermans MC, Beck J, Buckner B, Janick-Buckner D, Nettleton D, Scanlon MJ, Schnable PS: Global gene expression analysis of the shoot apical meristem of maize (Zea mays L.). Plant J. 2007, 52: 391-404. 10.1111/j.1365-313X.2007.03244.x.
Barbazuk WB, Emrich SJ, Chen HD, Li L, Schnable PS: SNP discovery via 454 transcriptome sequencing. Plant J. 2007, 51: 910-918. 10.1111/j.1365-313X.2007.03193.x.
Novaes E, Drost DR, Farmerie WG, Pappas GJ, Grattapaglia D, Sederoff RR, Kirst M: High-throughput gene and SNP discovery in Eucalyptus grandis, an uncharacterized genome. BMC Genomics. 2008, 9: 312-10.1186/1471-2164-9-312.
Barakat A, DiLoreto DS, Zhang Y, Smith C, Baier K, Powell WA, Wheeler N, Sederoff R, Carlson JE: Comparison of the transcriptomes of American chestnut (Castanea dentata) and Chinese chestnut (Castanea mollissima) in response to the chestnut blight infection. BMC Genomics. 2009, 9: 51-
Alagna F, D'Agostino N, Torchia L, Servili M, Rao R, Pietrella M, Giuliano G, Chiusano ML, Baldoni L, Perrotta G: Comparative 454 pyrosequencing of transcripts from two olive genotypes during fruit development. BMC Genomics. 2009, 10: 399-10.1186/1471-2164-10-399.
Trick M, Long Y, Meng J, Bancroft I: Single nucleotide polymorphism (SNP) discovery in the polyploid Brassica napus using Solexa transcriptome sequencing. Plant Biotechnol J. 2009, 7: 334-346. 10.1111/j.1467-7652.2008.00396.x.
Wang W, Wang Y, Zhang Q, Qi Y, Guo D: Global characterization of Artemisia annua glandular trichome transcriptome using 454 pyrosequencing. BMC Genomics. 2009, 10: 465-10.1186/1471-2164-10-465.
Christenson EA: Phalaenopsis. 2001, Portland, OR: Timber Press
Lin S, Lee HC, Chen WH, Chen CC, Kao YY, Fu YM, Chen YH, Lin TY: Nuclear DNA contents of Phalaenopsis species and Doritis pulcherrima. J Am Soc Hortic Sci. 2001, 126: 195-199.
Kao YY, Chang SB, Lin TY, Hsieh CH, Chen YH, Chen WH, Chen CC: Differential accumulation of heterochromatin as a cause for karyotype variation in Phalaenopsis orchids. Ann Bot. 2001, 87: 387-395. 10.1006/anbo.2000.1348.
Belarmino MM, Mii M: Agrobacterium-mediated genetic transformation of a Phalaenopsis orchid. Plant Cell Rep. 2000, 19: 435-442. 10.1007/s002990050752.
Mishiba K, Chin DP, Mii M: Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep. 2005, 24: 297-303. 10.1007/s00299-005-0938-8.
Chan YL, Lin KH, Liao LJ, Chen WH, Chan MT: Gene stacking in Phalaenopsis orchid enhances dual tolerance to pathogen attack. Transgenic Res. 2005, 14: 279-288. 10.1007/s11248-005-0106-5.
Lu HC, Chen HH, Tsai WC, Chen WH, Su HJ, Chang DCN, Yeh HH: Strategies for functional validation of genes involved in reproductive stages of orchids. Plant Physiol. 2007, 143: 558-569.
Fu CH, Chen YW, Hsiao YY, Pan ZJ, Liu ZJ, Huang YM, Tsai WC, Chen HH: OrchidBase: A collection of sequences of transcriptome derived from orchids. Plant Cell Physiol. 2011, 52: 238-243. 10.1093/pcp/pcq201.
Swarbreck D, Wilks C, Lamesch P, Berardini T, Garcia-Hernandez M, Foerster H, Li D, Meyer T, Muller R, Ploetz L, Radenbaugh A, Singh S, Swing V, Tissier C, Zhang P, Huala E: The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res. 2007, gkm965-
Blanc G, Wolfe KH: Widespread Paleopolyploidy in Model Plant Species Inferred from Age Distributions of Duplicate Genes. Plant Cell. 2004, 16: 1667-1678. 10.1105/tpc.021345.
Cui L, Wall PK, Leebens-Mack JH, Lindsay BG, Soltis DE, Doyle JJ, Soltis PS, Carlson JE, Arumuganathan K, Barakat A, Albert VA, Ma H, dePamphilis CW: Widespread genome duplications throughout the history of flowering plants. Genome Res. 2006, 16: 738-749. 10.1101/gr.4825606.
Libault M, Joshi T, Benedito VA, Xu D, Udvardi MK, Stacey G: Legume transcription factor genes: What makes legumes so special?. Plant Physiol. 2009, 151: 991-1001. 10.1104/pp.109.144105.
Nelson DR, Schuler MA, Paquette SM, Werck-Reichhart D, Bak S: Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol. 2004, 135: 756-772. 10.1104/pp.104.039826.
Nelson DR, Ming R, Alam M, Schuler MA: Comparison of cytochrome P450 genes from six plant genomes. Trop Plant Biol. 2008, 1: 216-235. 10.1007/s12042-008-9022-1.
Hsu YF, Tzeng JD, Liu MC, Yei FL, Chung MC, Wang CS: Identification of anther-specific/predominant genes regulated by gibberellin during development of lily anthers. J Plant Physiol. 2008, 165: 553-563. 10.1016/j.jplph.2007.01.008.
Van de Peer Y, Maere S, Meyer A: The evolution significance of ancient genome duplications. Nat Rev Genet. 2009, 10: 725-732. 10.1038/nrg2600.
Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Andrew H. Paterson AH, Zheng C, Sankoff D, dePamphilis CW, Wall PK, Soltis PS: Polyploidy and angiosperm diversification. Am J Bot. 2009, 96: 336-348. 10.3732/ajb.0800079.
Li Z, Thomas TL: PEI1, an embryo-specific zinc finger protein gene required for heart-stage embryo formation in Arabidopsis. Plant Cell. 1998, 10: 383-398.
Sonoda Y, Yao S-G, Sako K, Sato T, Kato W, Ohto M-a, Ichikawa T, Matsui M, Yamaguchi J, Ikeda A: SHA1, a novel RING finger protein, functions in shoot apical meristem maintenance in Arabidopsis. Plant J. 2007, 50: 586-596. 10.1111/j.1365-313X.2007.03062.x.
Ko JH, Yang SH, Han KH: Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. 2006, 47: 343-355. 10.1111/j.1365-313X.2006.02782.x.
Hilbricht T, Salamini F, Bartels D: CpR18, a novel SAP-domain plant transcription factor, binds to a promoter region necessary for ABA mediated expression of the CDeT27-45 gene from the resurrection plant Craterostigma plantagineum Hochst. Plant J. 2002, 31: 293-303. 10.1046/j.1365-313X.2002.01357.x.
Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K: OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33: 751-763. 10.1046/j.1365-313X.2003.01661.x.
Qin Q-l, Liu J-g, Zhang Z, Peng R-h, **ong A-s, Yao Q-h, Chen J-m: Isolation, optimization, and functional analysis of the cDNA encoding transcription factor OsDREB1B in Oryza Sativa L. Mol Breeding. 2007, 19: 329-340. 10.1007/s11032-006-9065-7.
Gutterson N, Reuber TL: Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol. 2004, 7: 465-471. 10.1016/j.pbi.2004.04.007.
Agarwal P, Agarwal P, Reddy M, Sopory S: Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25: 1263-1274. 10.1007/s00299-006-0204-8.
Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker W, Gerentes D, Perez P, Smyth DR: AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 1996, 8: 155-168.
Chen WH, Tseng YC, Liu YC, Chuo CM, Chen PT, Tseng KM, Yeh YC, Ger MJ, Wang HL: Cool night temperature induces spike emergence and affects photosynthetic efficiency and metabolizable carbohydrate and organic acid pools in Phalaenopsis aphrodite. Plant Cell Rep. 2008, 27: 1667-1675. 10.1007/s00299-008-0591-0.
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25: 3389-3402. 10.1093/nar/25.17.3389.
Acknowledgements
We thank Dr. Michel Delseny for providing critical comments on the manuscript. We also thank Miss Laura Smales for detailed editing the manuscript. We thank Dr. David T.H. Ho for his long-term support of our work on orchid genomics. This work was supported by the National Science Council, Taiwan (grant no. NSC97-2317-B-024-001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Authors' contributions
YYH conceived the study and design, participated in the library construction and data analysis. YWC performed the design of the bioinformatic analyses. SCH carried out RNA extraction and cDNA synthesis. ZJP contributed to the sample collection. CHF constructed the platform for displaying metabolic pathway. WHC suggested and offered the orchid materials. WCT participated in the design and coordination, and drafted the manuscript. HHC initiated the project, contributed to the experimental design and edited the manuscript. All authors read and approved the final manuscript.
Yu-Yun Hsiao, Yun-Wen Chen contributed equally to this work.
Electronic supplementary material
12864_2011_3496_MOESM1_ESM.DOC
Additional file 1:Length distribution of assembled contigs and singletons. This table summarizes the number of contigs and singletons in different length distribution. (DOC 39 KB)
12864_2011_3496_MOESM2_ESM.DOC
Additional file 2:Summary of component reads per assembly. This table summarizes the number of component reads assembled into contigs. (DOC 27 KB)
12864_2011_3496_MOESM3_ESM.DOC
Additional file 3:Gene Families identified by BLAST annotation of Phalaenopsis transcriptome. This table summarizes the BLAST results of all Unigenes against Arabidopsis proteome and then categorized by Arabidopsis gene families. (DOC 151 KB)
12864_2011_3496_MOESM4_ESM.DOC
Additional file 4:Expressed sequence tags with substantial similarity to terpenoid backbone biosynthetic genes. This table summarizes the number of unigenes and reads in each step of terpenoid biosynthetic pathway. (DOC 128 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hsiao, YY., Chen, YW., Huang, SC. et al. Gene discovery using next-generation pyrosequencing to develop ESTs for Phalaenopsis orchids. BMC Genomics 12, 360 (2011). https://doi.org/10.1186/1471-2164-12-360
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2164-12-360