Abstract
Background
Induced pluripotent stem cells (iPSC) are generated by reprogramming somatic cells into embryonic like state (ESC) using defined factors. There is great interest in these cells because of their potential for application in regenerative medicine.
Results
iPSC reprogrammed from murine tail tip fibroblasts were exposed to retinoic acid alone (RA) or in combination with TGF-β1 and 3, basic fibroblast growth factor (bFGF) or bone morphogenetic protein 2 (BMP-2). The resulting cells expressed selected putative mesenchymal stem cells (MSCs) markers; differentiated toward osteoblasts and adipocytic cell lineages in vitro at varying degrees. TGF-beta1 and 3 derived-cells possessed higher potential to give rise to osteoblasts than bFGF or BMP-2 derived-cells while BMP-2 derived cells exhibited a higher potential to differentiate toward adipocytic lineage. TGF-β1 in combination with RA derived-cells seeded onto HA/TCP ceramics and implanted in mice deposited typical bone. Immunofluorescence staining for bone specific proteins in cell seeded scaffolds tissue sections confirmed differentiation of the cells into osteoblasts in vivo.
Conclusions
The results demonstrate that TGF-beta family of proteins could potentially be used to generate murine iPSC derived-cells with potential for osteoblasts differentiation and bone formation in vivo and thus for application in musculoskeletal tissue repair and regeneration.
Similar content being viewed by others
Background
Induced pluripotent stem cells (iPSC) have generated hope and excitement because of the potential they possess in regenerative medicine. Since the discovery by Yamanaka and colleagues that somatic cells can be reprogrammed to embryonic like state (ESC), numerous reports have emerged focusing on methods to generate them efficiently and safely for future clinical applications [1–7]. Some progress has been made toward development of techniques for generating safer iPSC; for example; generation of virus free iPSC, thus avoiding potential of viral effects on tumor formation, use of protein factors to reprogram somatic cells and reprogramming without using oncogenic factors [5–11]. In addition, efficiencies in reprogramming somatic cells have improved [12–15]. Although more work is still needed to get iPSC closer to clinical application, it is also critical to begin to understand factors that play a role in directing differentiation of iPSC to various cell lineages prior to clinical application. In this regard, several studies focusing on methods to direct iPSC to specific cell lineages are active areas of investigation [16–19]. These approaches emulate methods developed for directing ESC to various lineages [20–22]. Most studies have focused on directing ESC or iPSC to hematopoietic and or neural cells lineages [16–19]. As a proof of concept, iPSC were generated from a humanized mouse model of sickle cell anemia followed by correction of the sickle cell defect. iPSC with the corrected gene were then directed to hematopoietic cell lineage and given back to the somatic cell donor [18]. This proof of concept demonstrated that it was possible to direct iPSC to hematopoietic lineage efficiently at least for murine iPSC.
Directing human ESC or iPSC to neural lineages has also gained some success [17, 20, 23, 24]; Wernig and colleagues showed that iPS-cell-derived dopaminergic neurons could alleviate the disease phenotype in a rat model of Parkinson’s disease [17]. Differentiation of human embryonic stem cells (hESC) and or human induced pluripotent stem cells (hiPSC) to mesenchymal cell lineage have also been reported [53, 54]. After 28 days of incubation, cells were stained in Alizarin Red S solution and examined under a light microscope. Alizarin deposits were extracted with 10% acetic acid and used for quantification of mineralization.
Adipogenic differentiation
For adipogenic differentiation, iPSC-derived cells were plated in 12 well plates in adipogenic medium at a cell density of 5 × 103 cells per well. The adipogenic medium was composed of DMEM with high glucose supplemented with 10% FBS, 0.1 mM indomethacin, 0.5 mM isobutylmethylxanthine (Sigma-Aldrich), and 10-6 M dexamethasone. The media were replaced every 3 days for 28 days. Adipogenic differentiation was assessed by Oil Red O staining at 3 weeks after initial adipogenic induction. For Oil Red O staining, the cells were rinsed in PBS and fixed in 10% formalin followed by incubation of the cells in 2% (wt/vol) Oil Red O reagent for 5 minutes at room temperature, examined under light microscope and photographed. The cells were suspended in 0.5 ml of isopropanol to extract Oil Red O for quantification of the level of adipogenic differentiation.
In vivo bone formation
The Institutional Animal Care and Use Committee of Penn State University College of Medicine approved all animal procedures; all animal experiments were carried out following the approved protocol. Retinoic acid alone or in combination with TGF-beta1 iPSC derived cells were trypsinized and seeded onto HA/TCP ceramic scaffolds at 5 × 106 cells/mL. Cells were allowed to attach to the ceramics for 2 h at 37°C prior to implantation in animals. Cell seeded Scaffolds were implanted subcutaneously onto the backs of thymic SCID mice. Five weeks after implantation; animals were sacrificed and the scaffolds were harvested.
Histological analysis
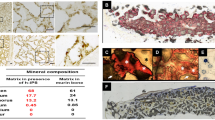
For histological analysis, methods described previously were used [53]. Briefly, HA/TCP ceramic scaffolds seeded or not seeded with iPSC-derived cells and retrieved from recipient mice were fixed in freshly prepared 4% paraformaldehyde in PBS, containing 10% sucrose. Following fixation, the scaffolds were decalcified and embedded in paraffin. Ten micron tissue sections were cut, prepared for histological analysis and stained with H and E. Tissue sections were also stained by a modified Masson Trichrome Staining to demonstrate collagen synthesis [55].
Immunofluorescence for Osteocalcin and Dentin matrix protein
Cryosections prepared from the ceramic scaffolds seeded or not seeded with cells were retrieved from recipient mice at 5 weeks following implantation. Tissue sections were fixed in cold acetone for 5 minutes and treated with 10% goat serum, followed by treatment with a polyclonal antibody specific for Osteocalcin (1:20 Millipore) or DMP-1 (1:50 RDI). Tissue sections made from murine cortical bone were treated similarly. For visualization, sections were treated with the secondary rabbit anti-rat antibodies conjugated with either FITC (Millipore) or Rhodamine (Santa Cruz, Santa Cruz CA) at a concentration of 1:1000 and 1:500 respectively.
Gene expression analysis
Gene expression analysis of osteogenic and adipogenic associated genes were performed as described previously [53]. Triplicate PCR reactions were carried out.
Statistical analysis
Statistical analysis was carried out using SPSS® software (SPSS, Chicago, IL). One-way ANOVA with a Tukey’s post-hoc analysis was used to evaluate for differences in growth factors treated and untreated samples for osteogenic and adipogenic differentiation and marker expression. Significance was set at P < 0.05.
References
Takahashi K, Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006, 126 (4): 663-676.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007, 131 (5): 861-872.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R: Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007, 318 (5858): 1917-1920.
Abujarour R, Ding S: Induced pluripotent stem cells free of exogenous reprogramming factors. Genome Biol. 2009, 10 (5): 220-
Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA: Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009, 324 (5928): 797-801.
Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K: Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009, 458 (7239): 771-775.
Gonzalez F, Barragan Monasterio M, Tiscornia G, Montserrat Pulido N, Vassena R, Batlle Morera L, Rodriguez Piza I, Izpisua Belmonte JC: Generation of mouse-induced pluripotent stem cells by transient expression of a single nonviral polycistronic vector. Proc Natl Acad Sci U S A. 2009, 106 (22): 8918-8922.
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S: Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008, 322 (5903): 949-953.
Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R: Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009, 4 (6): 472-476.
Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y: Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009, 4 (5): 381-384.
Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A: Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010, 7 (5): 618-630.
Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S: A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008, 2 (6): 525-528.
Mali P, Chou BK, Yen J, Ye Z, Zou J, Dowey S, Brodsky RA, Ohm JE, Yu W, Baylin SB: Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010, 28 (4): 713-720.
Maherali N, Hochedlinger K: Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr Biol. 2009, 19 (20): 1718-1723.
Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S: Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010, 6 (1): 71-79.
Xu D, Alipio Z, Fink LM, Adcock DM, Yang J, Ward DC, Ma Y: Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci U S A. 2009, 106 (3): 808-813.
Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R: Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A. 2008, 105 (15): 5856-5861.
Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM: Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007, 318 (5858): 1920-1923.
Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, Consiglio A, Castella M, Rio P, Sleep E: Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009, 460 (7251): 53-59.
Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC: Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008, 26 (1): 55-63.
Kim DS, Kim JY, Kang M, Cho MS, Kim DW: Derivation of functional dopamine neurons from embryonic stem cells. Cell Transplant. 2007, 16 (2): 117-123.
Lamba DA, Gust J, Reh TA: Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009, 4 (1): 73-79.
Sharp J, Keirstead HS: Stem cell-based cell replacement strategies for the central nervous system. Neurosci Lett. 2009, 456 (3): 107-111.
Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O: Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005, 25 (19): 4694-4705.
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Lam FF, Kang S, **a JC, Lai WH, Au KW: Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010, 121 (9): 1113-1123.
Wu R, Gu B, Zhao X, Tan Z, Chen L, Zhu J, Zhang M: Derivation of multipotent nestin(+)/CD271 (-)/STRO-1 (-) mesenchymal-like precursors from human embryonic stem cells in chemically defined conditions. Hum Cell. 2011, ePub
Barberi T, Willis LM, Socci ND, Studer L: Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005, 2 (6): e161-
Villa-Diaz LG, Brown SE, Liu Y, Ross AM, Lahann J, Parent JM, Krebsbach PH: Derivation of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells Cultured on Synthetic Substrates. Stem Cells. 2012, 30 (6): 1174-1181.
Bilousova G, du Jun H, King KB, De Langhe S, Chick WS, Torchia EC, Chow KS, Klemm DJ, Roop DR, Majka SM: Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells. 2011, 29 (2): 206-216.
Jukes JM, Both SK, Leusink A, Sterk LM, van Blitterswijk CA, de Boer J: Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A. 2008, 105 (19): 6840-6845.
Li F, Bronson S, Niyibizi C: Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem. 2010, 109 (4): 643-652.
Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L: Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007, 13 (5): 642-648.
Boyde A, Jones SJ: Bone modelling in the implantation bed. J Biomed Mater Res. 1985, 19 (3): 199-224.
Feng JQ, Ward LM, Liu S, Lu Y, **e Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S: Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006, 38 (11): 1310-1315.
Jung Y, Bauer G, Nolta JA: Induced Pluripotent Stem Cell - Derived Mesenchymal Stem Cells: Progress Toward Safe Clinical Products. Stem Cells. 2011, 30 (1): 42-47.
zur Nieden NI, Price FD, Davis LA, Everitt RE, Rancourt DE: Gene profiling on mixed embryonic stem cell populations reveals a biphasic role for beta-catenin in osteogenic differentiation. Mol Endocrinol. 2007, 21 (3): 674-685.
Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH: Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004, 10 (1): 55-63.
Toh WS, Yang Z, Liu H, Heng BC, Lee EH, Cao T: Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells. 2007, 25 (4): 950-960.
Mrugala D, Dossat N, Ringe J, Delorme B, Coffy A, Bony C, Charbord P, Haupl T, Daures JP, Noel D: Gene expression profile of multipotent mesenchymal stromal cells: Identification of pathways common to TGFbeta3/BMP2-induced chondrogenesis. Cloning Stem Cells. 2009, 11 (1): 61-76.
Reddi AH: Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998, 16 (3): 247-252.
Huang HY, Hu LL, Song TJ, Li X, He Q, Sun X, Li YM, Lu HJ, Yang PY, Tang QQ: nvolvement of cytoskeleton-associated proteins in the commitment of C3H10T1/2 pluripotent stem cells to adipocyte lineage induced by BMP2/4. Mol Cell Proteomics. 2011, 10 (1): M110 002691-
Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ: BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009, 106 (31): 12670-12675.
Tang QQ, Otto TC, Lane MD: Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2004, 101 (26): 9607-9611.
Butterwith SC, Wilkie RS, Clinton M: Treatment of pluripotential C3H 10 T1/2 fibroblasts with bone morphogenetic protein-4 induces adipocyte commitment. Biochem Soc Trans. 1996, 24 (2): 163S-
Wang EA, Israel DI, Kelly S, Luxenberg DP: Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3 T3 cells. Growth Factors. 1993, 9 (1): 57-71.
Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G: Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993, 12 (10): 871-880.
Date T, Doiguchi Y, Nobuta M, Shindo H: Bone morphogenetic protein-2 induces differentiation of multipotent C3H10T1/2 cells into osteoblasts, chondrocytes, and adipocytes in vivo and in vitro. J Orthop Sci. 2004, 9 (5): 503-508.
Watabe T, Miyazono K: Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009, 19 (1): 103-115.
Kimelman D: Mesoderm induction: from caps to chips. Nat Rev Genet. 2006, 7 (5): 360-372.
Taipaleenmaki H, Harkness L, Chen L, Larsen KH, Saamanen AM, Kassem M, Abdallah BM: The Cross-talk Between TGF-beta1 and Dlk1 Mediates Early Chondrogenesis During Embryonic Endochondral Ossification. Stem Cells. 2011, 30 (2): 304-313.
Duplomb L, Dagouassat M, Jourdon P, Heymann D: Differentiation of osteoblasts from mouse embryonic stem cells without generation of embryoid body. In Vitro Cell Dev Biol Anim. 2007, 43 (1): 21-24.
Wang X, Li F, Niyibizi C: Progenitors systemically transplanted into neonatal mice localize to areas of active bone formation in vivo: implications of cell therapy for skeletal diseases. Stem Cells. 2006, 24 (8): 1869-1878.
Li F, Wang X, Niyibizi C: Distribution of single-cell expanded marrow derived progenitors in a develo** mouse model of osteogenesis imperfecta following systemic transplantation. Stem Cells. 2007, 25 (12): 3183-3193.
Li F, Wang X, Niyibizi C: Bone marrow stromal cells contribute to bone formation following infusion into femoral cavities of a mouse model of osteogenesis imperfecta. Bone. 2010, 47 (3): 546-555.
Phillips CL, Bradley DA, Schlotzhauer CL, Bergfeld M, Libreros-Minotta C, Gawenis LR, Morris JS, Clarke LL, Hillman LS: Oim mice exhibit altered femur and incisor mineral composition and decreased bone mineral density. Bone. 2000, 27 (2): 219-226.
Acknowledgement
This work was supported by a grant from NIH/NIAMS number 1R21AR059383.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Dr. FL performed the experiments and assisted in data interpretation and manuscript preparation. Dr. CN formulated the project idea, interpreted data and assisted in manuscript preparation and submission. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, F., Niyibizi, C. Cells derived from murine induced pluripotent stem cells (iPSC) by treatment with members of TGF-beta family give rise to osteoblasts differentiation and form bone in vivo. BMC Cell Biol 13, 35 (2012). https://doi.org/10.1186/1471-2121-13-35
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2121-13-35