Abstract
Synthesis of proton-conducting materials based on track-etched membranes from polyvinylidene fluoride and sulfonated cross-linked polystyrene is described. The synthesis has been carried out by filling the pores of the original or gamma-irradiated track-etched membrane by copolymerization of styrene/divinylbenzene followed by sulfonation of polystyrene with chlorosulfonic acid. The resulting membranes have been studied by scanning electron microscopy and ATR IR spectroscopy. Membrane ionic conductivity, hydrogen gas permeability, ion-exchange capacity, and water absorption were measured. The ionic conductivity at 30°C reaches 51.7 mS/cm, which is almost three times higher than for Nafion®212 membranes; however, the gas permeability of the obtained materials also increases simultaneously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
One of the ways to obtain ion-exchange membranes is the polymerization of functional monomers inside materials with disordered porosity. In the English literature, the materials obtained by this method are called pore filling membranes [1–6]. The pore-filling polymer provides the functional properties of the synthesized material, while the porous matrix determines the membrane thickness and its mechanical properties. Polymerization inside the pores can be initiated both by the addition of traditional chemical initiators, such as benzoyl peroxide [7], and by irradiation, for example, UV [8], gamma [9], or beta radiation [10].
However, it seems more promising to use materials with ordered porosity, which is predominantly oriented perpendicular to the membrane surface. This should optimize the arrangement of conduction channels in the synthesized material. From this point of view, it is advisable to use track-etched membranes as a porous matrix, which are films with through pores, the shape of which is close to cylindrical [11]. Track-etched membranes are usually used for filtering various media [12, 13] or as a convenient object for studying the mechanism of diffusion of ions and molecules [13, 14].
The most popular synthesis approach of ion-conductive membranes based on track membranes is the radiation grafting of styrene in the latent track of films treated with a flow of heavy ions followed by polystyrene sulfonation [15–18]. This approach is based on the post-effect and consists in initiating the copolymerization of styrene by trapped radicals in the structure of irradiated (not having been etched) films. Recently, an ion-conducting membranes synthesis approach has been developed that consists in expanding the latent track of polyethylene terephthalate films due to UV irradiation to subnanometer sizes [19]. Carboxyl groups are responsible for the ionic conductivity in the track, providing a conductivity on the order of 2–3 mS/cm in the K form (≈0.02 M KCl, room temperature, difference method).
The aim of this work was to synthesize proton-conducting materials by polymerization of styrene in polyvinylidene fluoride (PVDF) track-etched membranes followed by sulfonation and to study their properties. To the best of our knowledge, track-etched membranes have not previously been used to obtain ion-conducting membranes by pore filling approach. To improve the bonding of the polymer film, a part of track-etched membranes were additionally activated by gamma radiation before polymerization.
EXPERIMENTAL
Reagents. Styrene (99%, extra pure, stabilized, Acros Organics); isopropanol (Khimmed, chemically pure grade, Russian State Standard); Ar (99.993%, Air Liquide); 1,2-dichloroethane (Khimmed, chemically pure grade); chlorosulfonic acid (99%, Sigma-Aldrich); HCl, NaCl, NaOH, anhydrous CaCl2 (Khimmed, chemically pure grade), Nafion® 212 (The Chemours Company FC, USA) were used in the experiments. Track-etched membranes were gamma-irradiated with a Cs-137 source (source activity 1.8 Gy/min) until a dose of 20 kGy was accumulated.
Styrene was purified from the inhibitor by treatment with sodium hydroxide for 24 h and three times washed with distilled water, then dried over anhydrous calcium chloride for 24 h, and distilled at 70°C under vacuum at a residual pressure of 60 mm Hg. Vacuum was created using a PC 3001 VARIO PRO vacuum pump (Vacuumbrand, Germany) with a CVC 3000 pressure controller. Divinylbenzene was purified by passing it through a column packed with a inhibitor remover (Sigma-Aldrich) to remove 4-tert-butylcatechol.
Production of track-etched membranes. The membranes were made from Solef 1008 polymer polyvinylidene fluoride film by exposure to heavy ions in a charged particle accelerator followed by chemical treatment. Exposure was carried out with beams of accelerated Xe ions with a density of 1 × 108 cm–2. Chemical treatment was carried out in the following order: etching, pre-washing after etching, finishing washing after etching, bleaching, pre-washing after bleaching, finishing washing after bleaching, and drying. The exposed film was etched in a solution containing sodium hydroxide with a mass fraction of 13%, sodium chloride with a mass fraction of 15%, and potassium permanganate with a mass fraction of 20%. The operating temperature of the etching solution corresponded to the boiling point at normal pressure (about 110°C). The duration of etching was 125 min.
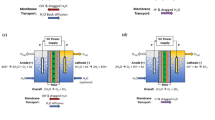
Synthesis of polymer composite by pore filling. For the synthesis of the composite by pore filling, two types of materials were used: the original track-etched membrane and the gamma-irradiated one. The track-etched membrane was placed between sheets of cellophane film and impregnated with a monomer mixture consisting of styrene and divinylbenzene used as a crosslinking agent. Benzoyl peroxide (20 g/L mixture) was used to initiate polymerization. Then the films were clamped between steel plates and placed in an oven at 120°C for 2 hours. After that, the films were separated and washed in 1,2-dichloroethane, followed by drying in air. The resulting composites were sulfonated in a 2% (vol) solution of chlorosulfonic acid in 1,2‑dichloroethane for 3 h. After sulfonation, the films were washed in isopropanol, dried in air, and hydrated in distilled water at 80–90°C 4 h. The obtained composite membranes based on the initial track-etched membrane are further designated as PF (Pore-Filled), and those based on the gamma-irradiated track-etched membrane are designated as PF-g.
Material characterization. To assess the change in membrane size during hydration, a square piece of wet membrane 4 × 4 cm2 in size was placed between sheets of filter paper to remove excess moisture, and its exact dimensions were measured using a caliper. Then the membrane was placed in an oven for 2 h at 80°С and its dimensions were measured again. The degree of swelling during hydration (SD, %) was calculated as the ratio of the area of the wet membrane to the area of the dry membrane.
Other methods of membrane characterization were described in detail [20]. Below is only a brief description of the experiments, designations, and formulas used for the calculation. In all experiments, except for the study by scanning electron microscopy, the membranes were in the proton form.
The ionic conductivity (σ, mS/cm) of membranes in deionized water was measured in a two-electrode contact cell in the temperature range of 24–80°C using impedance spectroscopy on a P-40X potentiostat-galvanostat with an impedance measurement module (Elins, Russia).
The water uptake of membranes (WU, %) was determined from the weight loss of samples equilibrated with deionized water after drying in an oven at 80°C for 2 h.
The ion exchange capacity (IEC, mg-eq/g) was determined using acid-base titration and calculated on the dry membrane weight.
The degree of hydration (λ, the number of water molecules per sulfo group) was calculated from the ion exchange capacity and water uptake using the following formula:
where Mw is the molar weight of water.
Hydrogen permeability (P(H2), cm2/s) was measured in a two-section cell by the flow of humidified hydrogen according to the procedure described [21]. Before determining the gas permeability, the samples were equilibrated in a desiccator with a constant relative humidity of 95% for 48 hours.
Attenuated total reflectance Fourier transform infrared spectroscopy studies were performed on a Nicolet iS5 IR spectrometer (Thermo Fisher Scientific, USA) using a Quest Specac attachment with a diamond crystal (spectral range 500–4000 cm–1, 32 scans, resolution 2 cm–1). The OMNIC® IR software Advanced ATR correction was used to correct the resulting spectra (bounces number—of 1, angle of incidence of 45°, and refractive index of 1.5).
The membrane morphology in the cesium form and the distribution of elements were analyzed using a Tescan Amber scanning electron microscope (Czech Republic) equipped with an AZtec system (Oxford Instruments) for X-ray spectral microanalysis (XSMA).
RESULTS AND DISCUSSION
Microstructure and Composition of Membranes
After the pores are filled with cross-linked polystyrene, an intense peak appears in the IR spectra of the films in the region of 680–720 cm–1, corresponding to bending vibrations of C–H bonds in the aromatic ring, a series of peaks are observed at 1492, 1600 cm–1, corresponding to C–C stretching benzene ring, as well as a series of peaks are observed at 2847, 2921, 3058, 3083 cm–1 with a relatively low intensity, corresponding to stretching vibrations of C–H bonds in the aliphatic and aromatic parts of polystyrene molecules [22].
As a result of sulfonation of the obtained composites, the intense signal in the IR spectrum disappears in the region of 680–720 cm–1, well-resolved peaks appear at 574, 674, and 1000 cm–1, and the intensity of the broad signal increases in the region of 1100 cm–1, which overlaps with intense peaks of stretching vibrations of the C–F bonds of polyvinylidene fluoride. In particular, new signals in the region of 1000–1100 cm–1 correspond to symmetric and asymmetric stretching vibrations of sulfo groups. The listed new signals correspond to the vibrations of polystyrenesulfonic acid [23], which confirms the successful completion of the polystyrene sulfonation reaction. In addition, due to the hydration of sulfo groups, peaks appear that are characteristic of the bending and stretching vibrations of sorbed water with maxima at 1653 and 3390 cm–1, respectively. At the same time, the О–Н stretching vibration band manifests itself in the form of a wide halo, typical for systems with strong hydrogen bonds, such as hydrated protons.
Upon completion of sulfonation, a part of the formed polymer exfoliates from the membrane surface. This is due to the fact that the polymerization of the monomer mixture occurs both inside the pores and on the surface of the track-etched membrane, and bonding of polystyrene to the membrane surface is rather weak.
The original track-etched membrane has pores with a diameter of about 200 nm and a total porosity of about 23% (Fig. 2, 1, 2). It is possible to fill almost all tracks with polystyrene, which is especially clearly seen in SEM images obtained in the backscattered electron mode (Fig. 2, 4,7). Due to the content of cesium ions, lighter areas correspond to the obtained polystyrenesulfonic acid. However, the new polymer phase is present not only in the pores, but also forms agglomerates on the surface. A large proportion of these agglomerates are formed on the surface of the gamma-irradiated PF-g membrane. These data are consistent with the high content of sulfur and cesium in the membranes of PF-g (Table 1).
Physicochemical and Transport Properties of Membranes
The number of titratable acid groups in the initial track membrane is negligible (Table 2). After immersion in water, the matte track-etched membrane becomes transparent, which indicates that the pores are filled with water. At the same time, after contact with the filter paper, the water quickly come off from the track-etched, and, according to thermogravimetry data, the mass loss due to dehydration is only 0.6%. Thus, the initial track membrane contains an extremely low amount of functional (most likely, carboxyl) groups, and water is retained in it mainly due to capillary forces. Despite the above, the original track membrane has a conductivity of about 0.1 mS/cm, apparently due to the transfer of protons formed as a result of the dissociation of carboxyl groups along the water sorbed on the surface of the tracks. The described features distinguish the studied track-etched membrane from a similar material based on polyethylene terephthalate, which has a high ionic conductivity in salt solutions [24]. Apparently, this difference is associated with the chemical properties of polymers; unlike polyvinylidene fluoride, polyethylene terephthalate, when etched, gives a high content of carboxyl groups on the track surface.
The successful filling of tracks with sulfonated polystyrene is indicated by a significant decrease in the gas permeability of membranes relative to the initial track-etched membrane (Table 2); however, it remains somewhat higher than for Nafion® 212 membranes. Moreover, for PF-g membranes obtained from additionally irradiated film, the gas permeability was found to be noticeably higher than that for membranes from non-irradiated films.
As a result of gamma irradiation of the initial track membrane, both polystyrene grafting and some changes in the PVDF structure occur. In addition, as a result of heating at a temperature of 120°C (during the polymerization of styrene), both the partial destruction of the film itself and the polystyrene grafted onto its surface can also occur, which leads to a significant difference in its properties from those of polystyrene obtained in volume (tracks) of membranes (the latter is more gas-tight). To assess the influence of the base-film degradation effect under the influence of irradiation and heat treatment, we carried out an additional measurement of the gas permeability of three samples of the PVDF film 50 μm thick: initial, gamma-irradiated, gamma-irradiated and heated at 120°C, the values of which were (1.9 ± 0.2) × 10–9, (2.4 ± 0.2) × 10–9, and (2.4 ± 0.2) × 10–9 cm2/s, respectively. Gamma irradiation leads to some increase in permeability, but its contribution is quite small. Thus, it can be assumed that the increase in specific gas permeability is primarily due to an increase in the thickness of the PF-g membranes because of the deposition of a layer of polystyrene sulfate with high gas permeability on the surface, which is confirmed by a similar ratio of gas permeabilities and thicknesses of the PF-g and PF samples. That is, an additional loose layer of polystyrene sulfate on the surface of these membranes (Fig. 2) has practically no effect on the hydrogen flow through them.
The theoretical value of the ion-exchange capacity based on a dry membrane can be estimated based on the complete filling of the tracks with polystyrene with a density of 1.05 g/mol, which is then sulfonated to form polystyrenesulfonic acid with an equivalent mass of 184 g/mol. The theoretical value of the ion-exchange capacity for a track membrane with a porosity of 23% filled with sulfonated polystyrene is 1.29 mg-eq/g. The downward deviation of the observed values of the ion-exchange capacity of the PF membrane (Table 2) is explained by the fact that the degree of pore filling and sulfonation is less than 100%. The excess of the theoretically estimated value of PF-g membrane results from the additional polymerization of styrene on the surface of the films.
The values of proton conductivity at 30°С for the obtained membranes are 2–3 times higher than for Nafion®212 membranes, and this difference persists with increasing temperature. Moreover, for materials obtained from irradiated track-etched membranes, the conductivity is approximately one and a half times higher than for non-irradiated ones. The high conductivity of composite materials based on track-etched membranes obtained by radiation grafting onto a latent track was noted earlier [15–18]. It is due to the presence of 1D-cylindrical channels perpendicular to the plane of the membrane, filled with sulfonated styrene with a high degree of hydration. According to the theory of percolation [26], such a structure provides the shortest highly conductive diffusion path and gives an advantage over traditional membranes such as Nafion, a system of pores and channels in which has a 3D branched structure with a longer diffusion path. In addition, the high concentration of functional groups in sulfonated polystyrene determines its high water uptake and ionic conductivity. For example, with a twofold increase in the capacity of perfluorinated homogeneous membranes, their conductivity increases by three orders of magnitude [27]. Unfortunately, poor mechanical properties, including those in the hydration-dehydration cycle, do not make it possible to obtain membranes from pure polystyrene sulfate, which is usually combined with a plasticizer and fixed with a reinforcing mesh. In this case, their role is played by the matrix of the PVDF track membrane, which is not hydrated and limits changes in the volume of polystyrene sulfate.
Therefore, another important advantage of the materials obtained based on track membranes is a low increase in area upon swelling (Table 2). For membranes obtained by pore filling, it is extremely low relative to radiation-grafted membranes [28] and varies in the range of 17–27%. This parameter is important from the point of view of membrane stability in operation, for example, in fuel cells, since a significant change in size upon hydratation leads to exfoliation of the catalytic layer and a drop in performance.
CONCLUSIONS
Ion-conducting membranes based on a track-etched membrane made of PVDF and sulfonated cross-linked polystyrene have been synthesized by the pore-filling method. An ion-conducting membrane made from a non-irradiated track-etched membrane has an ion-exchange capacity of 1.09 mg-eq/g and a proton conductivity of 30.8 mS/cm at 30°C, which is almost twice as high as the conductivity of Nafion®212 membranes. A significant advantage of such membranes is a small change in area size during swelling. Additional gamma irradiation of the original film leads to better adhesion of polystyrene and a significant increase in proton conductivity. Given the high ionic conductivity and high selectivity, the developed materials may be of interest for applications such as electrodialysis, reverse electrodialysis, flow batteries, and others.
REFERENCES
H.-B. Song, D.-H. Kim, and M.-S. Kang, Membranes (Basel) 12, 196 (2022). https://doi.org/10.3390/membranes12020196
S. C. Yang, Y. W. Choi, J. Choi, N. Jeong, H. Kim, H. Jeong, S. Y. Byeon, H. Yoon, and Y. H. Kim, J. Memb. Sci. 584, 181 (2019). https://doi.org/10.1016/j.memsci.2019.04.075
V. Chavan, C. Agarwal, V. C. Adya, and A. K. Pandey, Water Res. 133, 87 (2018). https://doi.org/10.1016/j.watres.2018.01.023
X. **ao, M. A. Shehzad, A. Yasmin, Z. Zhang, X. Liang, L. Ge, J. Zhang, L. Wu, and T. Xu, J. Memb. Sci. 597, 117776 (2020). https://doi.org/10.1016/j.memsci.2019.117776
T. Yamaguchi, F. Miyata, and S. Nakao, J. Memb. Sci. 214, 283 (2003). https://doi.org/10.1016/S0376-7388(02)00579-3
N. Wang, S. Ji, J. Li, R. Zhang, and G. Zhang, J. Memb. Sci. 455, 113 (2014). https://doi.org/10.1016/j.memsci.2013.12.023
Y. Zhao, H. Yu, F. **e, Y. Liu, Z. Shao, and B. Yi, J. Power Sources 269, 1 (2014). https://doi.org/10.1016/j.jpowsour.2014.06.026
D. H. Kim, J. S. Park, M. Choun, J. Lee, and M. S. Kang, Electrochim. Acta 222, 212 (2016). https://doi.org/10.1016/j.electacta.2016.10.041
D. S. Hwang, T. A. Sherazi, J. Y. Sohn, Y. C. Noh, C. H. Park, M. D. Guiver, and Y. M. Lee, J. Memb. Sci. 495, 206 (2015). https://doi.org/10.1016/j.memsci.2015.07.067
M. M. Nasef, N. A. Zubir, A. F. Ismail, M. Khayet, K. Z. M. Dahlan, H. Saidi, R. Rohani, T. I. S. Ngah, and N. A. Sulaiman, J. Memb. Sci. 268, 96 (2006). https://doi.org/10.1016/j.memsci.2005.06.009
P. Y. Apel, O. V. Bobreshova, A. V. Volkov, V. V. Volkov, V. V. Nikonenko, I. A. Stenina, A. N. Filippov, Y. P. Yampolskii, and A. B. Yaroslavtsev, Membr. Membr. Technol. 1, 45 (2019). https://doi.org/10.1134/S2517751619020021
P. Y. Apel, S. Velizarov, A. V. Volkov, T. V. Eliseeva, V. V. Nikonenko, A. V. Parshina, N. D. Pismenskaya, K. I. Popov, and A. B. Yaroslavtsev, Membr. Membr. Technol. 4, 69 (2022). https://doi.org/10.1134/S2517751622020032
D. Y. Butylskii, N. D. Pismenskaya, P. Y. Apel, K. G. Sabbatovskiy, and V. V. Nikonenko, J. Memb. Sci. 635, 119449 (2021). https://doi.org/10.1016/j.memsci.2021.119449
D. Y. Butylskii, S. A. Mareev, V. V. Nikonenko, N. D. Pismenskaya, C. Larchet, L. Dammak, D. Grande, and P. Y. Apel, Pet. Chem. 56, 1006 (2016). https://doi.org/10.1134/S0965544116110037
S. ichi Sawada, M. Goto, H. Koshikawa, A. Kitamura, M. Higa, and T. Yamaki, Sep. Sci. Technol. 55, 2211 (2020). https://doi.org/10.1080/01496395.2019.1648510
V. Sproll, M. Handl, R. Hiesgen, K.A. Friedrich, T. J. Schmidt, and L. Gubler, J. Mater. Chem. A 5, 24826 (2017). https://doi.org/10.1039/c7ta07323b
K. K. Jana, O. Prakash, V. K. Shahi, D. K. Avasthi, and P. Maiti, ACS Omega 3, 917 (2017). https://doi.org/10.1021/acsomega.7b01635
T. Yamaki, Y. Kozone, A. Hiroki, K. Hosoi, M. Asano, H. Kubota, and M. Yoshida, Electrochemistry 75, 175 (2007). https://doi.org/10.5796/electrochemistry.75.175
P. Y. Apel, I. V. Blonskaya, O. M. Ivanov, O. V. Kristavchuk, N. E. Lizunov, A. N. Nechaev, O. L. Orelovich, O. A. Polezhaeva, and S. N. Dmitriev, Membr. Membr. Technol. 2, 98 (2020). https://doi.org/10.1134/S251775162002002X
D. V. Golubenko, V. R. Malakhova, P. A. Yurova, M. V. Evsiunina, and I. A. Stenina, Membr. Membr. Technol. 4, 305 (2022). https://doi.org/10.1134/S2517751622040035
I. A. Stenina, P. A. Yurova, T. S. Titova, M. A. Polovkova, O. V. Korchagin, V. A. Bogdanovskaya, and A. B. Yaroslavtsev, J. Appl. Polym. Sci. 138, 50644 (2021). https://doi.org/10.1002/app.50644
G. Socrates, Occup. Med. (Lond). 62, 347 (2001).
N. M. Shishlov and S. L. Khursan, J. Mol. Struct. 1123, 360 (2016). https://doi.org/10.1016/j.molstruc.2016.06.030
V. V. Sarapulova, E. L. Pasechnaya, V. D. Titorova, N. D. Pismenskaya, P. Yu. Apel, and V. V. Nikonenko, Membr. Membr. Technol. 2, 333 (2020). https://doi.org/10.1134/S2517751620050066
E. Y. Safronova, A. K. Osipov, and A. B. Yaroslavtsev, Pet. Chem. 58, 130 (2018). https://doi.org/10.1134/S0965544118020044
B. J. Sung and A. Yethiraj, Phys. Rev. Lett. 96, 1 (2006). https://doi.org/10.1103/PhysRevLett.96.228103
I. A. Prikhno, E. Y. Safronova, I. A. Stenina, P. A. Yurova, and A. B. Yaroslavtsev, Membr. Membr. Technol. 2, 265 (2020). https://doi.org/10.1134/s2517751620040095
S. Holmberg, J. H. Näsman, and F. Sundholm, Polym. Adv. Technol. 9, 121 (1998). https://doi.org/10.1002/(SICI)1099-1581(199802)9:2<121::AID-PAT724>3.0.CO;2-M
ACKNOWLEDGMENTS
Scanning electron microscopy studies were carried out using the equipment of the Center for Collective Use of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
Funding
The work was carried out within the framework of the VE-479 project “Development of scientific and practical foundations for the creation of a nanostructured membrane with high proton conductivity for low-temperature fuel cells.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Golubenko, D.V., Yurova, P.A., Desyatov, A.V. et al. Pore Filled Ion-Conducting Materials Based on Track-Etched Membranes and Sulfonated Polystyrene. Membr. Membr. Technol. 4, 398–403 (2022). https://doi.org/10.1134/S2517751622060026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2517751622060026