Abstract
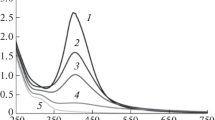
A study of the optical properties of iron nanoparticles prepared using the molecular-by-molecular method in the reactions of ion chemical and radiation-chemical reduction in inverse micelles, which were considered as microreactors, made it possible to reveal the features of the formation of metal nanoparticles (NPs) at various stages of physicochemical processes including a spontaneous formation of ordered spatial nanostructures in the postradiation period. The analysis of the spectral data obtained from the measurements in inverse micellar solutions (IMS), which were free of metal ions, provided important information on the evolution of colloid systems. After adding iron ions into an IMS, the Fe NP formation induced by self-assembly processes was detected via characteristic optical absorbance spectra. Based on the results obtained, some peculiarities of the self-assembly processes in IMSs occurring upon various ignition reactions of iron ion reduction and the Fe NP formation were discussed in the work.








Similar content being viewed by others
Notes
The idea of polarization of water in reverse micelles has been examined and discussed for a long time. See, for example, [20] – Editor.
REFERENCES
Petit, C., Lixon, P., and Pileni, M.-P., J. Phys. Chem., 1993, vol. 97, p. 12974.
Dokuchaev, A.A., Myasoedova, T.G., and Revina, A.A., High Energy Chem., 1997, vol. 31, no. 5, p. 316.
Egorova, E.M. and Revina, A.A., Colloids Surf., A, 2000, vol. 73, p. 87.
Tanasyuk, D.A., Revina, A.A., and Ermakov, V.I., Naukoemkie Tekhnol., 2012, vol. 13, no. 2, p. 9.
Revina, A.A., RF Patent 2312741, 2007.
Revina, A.A., RF Patent 2322327, 2008.
Revina A. A., Kuznetsov M. A., Chekmarev A. M., Boyakov E. E., Zolotarevskii V. I., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, Issue 1, pp 43–50.
Grzelczak, M., Vermant, J., Furst, E.M., et al., ACS Nano, 2010, vol. 4, no. 7, p. 3591.
Bukhtiyarov, V.I. and Slin’ko, M.G., Usp. Khim., 2001, vol. 70, no. 2, p. 167.
Revina, A.A., Kezikov, A.N., Kozlov, A.I, et al., RF Patent 2270831, 2006.
Revina, A.A., Prot. Met. Phys. Chem. Surf., 2009, vol. 45, no. 1, p. 54.
Revina, A.A. and Zaitsev, P.M., Russ. J. Electrochem., 2012, vol. 48, no. 4, p. 412.
Revina, A.A., Voropaeva, N.L., Mukhin, V.M., and Chekmar’, D.V., in Netraditsionnye prirodnye resursy, innovatsionnye tekhnologii i produkty (Non-Traditional Natural Resources, Innovative Technologies, and Products), Moscow: Russ. Acad. Natural Sci., 2016, issue 23, p. 30.
Shtykov, S.N. and Goryacheva, I.Yu., Opt. Spectrosc., 1997, vol. 83, no. 4, p. 645.
Voeikov, V.I., Trudy 4-ogo mezhdunarodnogo kongressa “Slabye i sverkhslabye polya i izlucheniya v biologii i meditsine” (Proc. 4th Int. Congress “Weak and Super-Weak Fields and Emissions for Biology and Medicine”), St. Petersburg, 2006, p. 46.
Voloshin, V.P., Zheligovskaya, E.A., Malenkov, G.G., Naberukhin, Yu.I., and Tytik, D.L., Ross. Khim. Zh., 2001, vol. 45, no. 3, p. 31.
Zheng, J.M., Wexler, A., and Pollack, G.H., J. Colloid Interface Sci., 2009, vol. 332, p. 511.
Tanasyuk, D.A., Tsetlin, V.V., Revina, A.A., and Ermakov, V.I., Naukoemkie Tekhnol., 2013, vol. 14, no. 1, p. 44.
Ermakov, V.I. and Revina, A.A., Obratnomitsellyarnye sistemy: Elektromagnitnye svoistva i struktura (Inverse-Micellar Systems: Electromagnetic Properties and Structure), Nizhny Novgorod: Nizhny Novgorod Institute of Management, Russian Presidential Academy of National Economy and Public Administration, 2017.
Nishirnoto, J., Iwamoto, E., Fujiwara, T., and Kurnarnaru, T., J. Chem. Soc. Faraday Trans., 1993, vol. 89, no. 3, pp. 535–538.
Revina, A.A., Daineko, S.V., Bol’shakova, A.N., et al., Naukoemkie Tekhnol., 2011, vol. 12, no. 1, p. 68.
Arslanov, V.V., Nanotekhnologiya. Kolloidnaya i supramolekulyarnaya khimiya. Entsiklopedicheskii spravochnik (Nanotechnology. Colloid and Supramolecular Chemistry. A Handbook), Moscow: LENAND, 2015.
ACKNOWLEDGMENTS
We are grateful to S.S. Abramchuk, associate professor of the Chair of Polymer and Crystal Physics of Department of Physics of Moscow State University, for assisting with TEM investigations.
The investigations were carried out with the use of a ULV-10-10-C-70 accelerator of the Center for Collective Use of Physical Methods of the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Science.
Funding
The work was supported partially by the Russian Foundation for Basic Research, project no. 16-03-00665.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Khozina
Rights and permissions
About this article
Cite this article
Revina, A.A., Suvorova, O.V., Pavlov, Y.S. et al. The Role of Self-Assembly Processes in the Formation of Iron Nanoparticles in Inverse Micelles. Prot Met Phys Chem Surf 55, 888–894 (2019). https://doi.org/10.1134/S2070205119040166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119040166