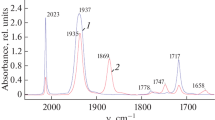
Abstract
Indolyl(thienyl)maleimides with terpyridine receptor in the bridge part have been synthesized. Irradiation of their toluene solutions with light at λ = 436 nm has led to the formation of cyclic isomers. The reverse ring opening reaction has occurred during the photolysis with visible light (λ > 500 nm). 1-(4-([2,2′:6′,2″-Terpyridin]-4′-yl)phenyl)derivatives have exhibited the properties of chromogenic naked-eye sensors to Fe2+ cations in acetonitrile solution, changing the solution color from bright-orange to red.
Similar content being viewed by others
References
Photon-Working Switches, Yokoyama, Y. and Nakatani, K., Eds., Tokyo: Springer, 2017. 464 p. doi https://doi.org/10.1007/978-4-431-56544-4
Molecular Switches, Feringa, B.L. and Browne, W.R., Eds., Weinheim: Wiley, 2011.
Minkin, V.I., Russ. Chem. Bull., 2008, vol. 57, no. 4, p. 687. doi https://doi.org/10.1007/s11172-008-0111-y
Andreasson, J. and Pischel, U., Chem. Soc. Rev., 2015, vol. 44, no. 5, p. 1053. doi https://doi.org/10.1039/c4cs00342j
Bazzicalupi, C., Bianchi, A., Garcia-Espana, E., and Delgado-Pinar, E., Inorg. Chim. Acta., 2014, vol. 417, p. 3. doi https://doi.org/10.1016/j.ica.2014.03.001
Velema, W.A., Szymanski, W., and Feringa, B.L., J. Am. Chem. Soc., 2014, vol. 136, no. 6, p. 2178. doi https://doi.org/10.1021/ja413063e
Zhang, J., Zou, Q., and Tian, H., Adv. Mater., 2013, vol. 25, no. 3, p. 378. doi https://doi.org/10.1002/adma.201201521
Natali, M. and Giordani, S., Chem. Soc. Rev., 2012, vol. 41, no. 10, p. 4010. doi https://doi.org/10.1039/C2CS35015G
Wang, G. and Zhang, J., J. Photochem. Photobiol., 2012, vol. 13, no. 4, p. 299. doi https://doi.org/10.1016/j.jphotochemrev.2012.06.002
Klajn, R., Chem. Soc. Rev., 2014, vol. 43, p. 148. doi https://doi.org/10.1039/C3CS60181A.
Photochromism: Molecules and Systems, Dürr, H. and Bouas-Laurent, H., Amsterdam: Elsevier, 2003, p. 314.
Yokoyama, Y., Chem. Rev., 2000, vol. 100, no. 5, p. 1717. doi https://doi.org/10.1021/cr980070c
Organic Photochemistry and Photobiology, Griesbeck, A., Oelgemöller, M., and Ghetti, F., Eds., Boca Raton; London; New York: CRC Press, 2012, p. 607.
Mukhopadhyay, A. and Moorthy, J.N., J. Photochem. Photobiol. (C), 2016, vol. 29, p. 73. doi https://doi.org/10.1016/j.jphotochemrev.2016.11.002
Zhao, X.Y. and Wang, M.Z., Eur. Polymer J., 2006, vol. 42, no. 2, p. 247. doi https://doi.org/10.1016/j.eurpolymj.2005.08.007
Irie, M., Chem. Rev., 2000, vol. 100, no. 5, p. 1685. doi https://doi.org/10.1021/cr980069d
Irie, M., Fukaminato, T., Matsuda, K., and Kobatake, S., Chem. Rev., 2014, vol. 114, no. 24, p. 12174. doi https://doi.org/10.1021/cr500249p
Lvov, A.G. and Shirinyan, V.Z., Chem. Heterocycl. Compd., 2016, vol. 52, no. 9, p. 658. doi https://doi.org/10.1007/s10593-016-1946-z
Harvey, E.C., Feringa, B.L., Vos, J.G., Browne, W.R., and Pryce, M.T., Coord. Chem. Rev., 2015, vols. 282–283, p. 77. doi 0.1016/j.ccr.2014.06.008
Zou, Q., **, J., Xu, B., Ding Li., and Tian, H., Tetrahedron, 2011, vol. 67, p. 915. doi https://doi.org/10.1016/j.tet.2010.12.019
Shepelenko, E.N., Karamov, O.G., Podshibyakin, V.A., Revinskii, Yu.V., Tikhomirova, K.S., Dubonosov, A.D., Bren, V.A., and Minkin, V.I., Arkivoc, 2017, no. 5, p. 196. doi https://doi.org/10.24820/ark.5550190.p010.262
Dubonosov, A.D., Bren, V.A., Minkin, V.I., Shepelenko, E.N., Tikhomirova, K.S., Starikov, A.G., and Revinskii, Yu.V., Tetrahedron, 2015, vol. 71, no. 46, p. 8817. doi https://doi.org/10.1016/j.tet.2015.09.030
Makarova, N.I., Levchenko, P.V., Tkachev, V.V., Shepelenko, E.N., Metelitsa, A.V., Rybalkin, V.P., Popova, L.L., Bren, V.A., Aldoshin, S.M., and Minkin, V.I., Russ. Chem. Bull., 2011, vol. 60, no. 6, p. 1090. doi https://doi.org/10.1007/s11172-011-0172-1
Makarova, N.I., Levchenko, P.V., Shepelenko, E.N., Metelitsa, A.V., Kozyrev, V.S., Rybalkin, V.P., Bren, V.A., and Minkin, V.I., Russ. Chem. Bull., 2011, vol. 60, no. 9, p. 1899. doi https://doi.org/10.1007/s11172-011-0286-5
Tikhomirova, K.S., Podshibyakin, V.A., Shepelenko, E.N., Revinskii, Yu.V., Dubonosov, A.D., and Bren, V.A., Chem. Heterocycl. Compd., 2018, vol. 54, no. 1, p. 32. doi https://doi.org/10.1007/s10593-018-2226-x
Liu, Z., He, W., and Guo, Z., Chem. Soc. Rev., 2013, vol. 42, no. 4, p. 1568. doi https://doi.org/10.1039/C2CS35363F
Kaur, N. and Kumar, S., Tetrahedron, 2011, vol. 67, no. 48, p. 9233. doi https://doi.org/10.1016/j.tet.2011.09.003
Mukherjee, S., Pal, P., Bar, M., and Baitalik, S., J. Chem. Sci., 2018, vol. 130, p. 84. doi https://doi.org/10.1007/s12039-018-1484-6
Liang, Z., Wang, C., Yang, J., Gao, H., Tian, Y., Tao, X., and Janiang, M., New J. Chem., 2007, vol. 31, p. 906. doi https://doi.org/10.1039/B701201M
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shepelenko, E.N., Podshibyakin, V.A., Tikhomirova, K.S. et al. Synthesis, Photo-, and Ionochromic Properties of Indolyl(thienyl)maleimides with Terpyridine Receptor. Russ J Gen Chem 89, 409–415 (2019). https://doi.org/10.1134/S1070363219030071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363219030071