Abstract
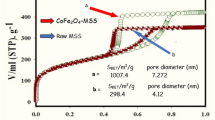
A modified polyacrylamide gel route with urea used as fuel was used to synthesize the MFe2O4 (M = Mg, Ca, Ba) iron oxides with high adsorption capacity for removal of Cd(II) from water treatment. The phase structure, functional group, porous structure and adsorption performance of MFe2O4 (M = Mg, Ca, Ba) iron oxides were characterized by X-ray-diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM), N2 adsorption–desorption isotherm and 721 spectrophotometer. The result indicates that the phase forming temperature of MFe2O4, the content of carbonate ion in MFe2O4 increases with the increasing of the radius of M-site metal ion. The Eg value, BET surface area and adsorption capacity for removal of Cd(II) from water treatment decreases with the increasing of the radius of M-site metal ion. The experimental isotherm of MFe2O4 (M = Mg, Ca, Ba) iron oxides were described by the Langmuir model. MgFe2O4 iron oxide exhibits a best adsorption performance for removal of Cd(II) from water treatment by the adsorption process, with a maximum adsorption capacity of ca. 221.36 mg/g than that of other iron oxides. The present synthetic route could be possibly extended to synthesize other porous metal oxide materials with special porous structures for various applications.






Similar content being viewed by others
REFERENCES
Z. Song, X. Chen, X. Gong, et al., Opt. Mater. 100, 109642 (2020).
M. Sharma, M. Poddar, Y. Gupta, et al., Mater. Today Chem. 17, 100336 (2020).
P. S. Gordienko, I. A. Shabalin, S. B. Yarusova, et al., Russ. J. Phys. Chem. A 93, 2284 (2019).
M. F. Cheira, M. N. Rashed, A. E. Mohamed, et al., Mater. Today Chem. 14, 100176 (2019).
X. Song, F. Ke, C. Ge, et al., Russ. J. Phys. Chem. A 93, 522 (2019).
D. Dutta, S. K. Roy, B. Das, and A. K. Talukdar, Russ. J. Phys. Chem. A 92, 976 (2018).
W. W. Aji and E. Suharyadi, Mater. Sci. Forum 901, 142 (2017).
V. Srivastava, Y. C. Sharma, and M. Sillanpää, Appl. Surf. Sci. 338, 42 (2015).
S. M. Yakout, M. R. Hassan, and M. I. Aly, Water Sci. Technol. 77, 2714 (2018).
J. Nonkumwong, S. Ananta, and L. Srisombat, RSC Adv. 6, 47382 (2016).
D. Kang, X. Yu, M. Ge, and W. Song, Microporous Mesoporous Mater. 207, 170 (2015).
S. Shi, Q. Dong, Y. Wang, X. Zhang, et al., Sep. Purif. Technol. 266, 118584 (2021).
M. Kaur, N. Kaur, K. Jeet, and P. Kaur, Ceram. Int. 41, 13739 (2015).
H. Kenfoud, N. Nasrallah, O. Baaloudj, et al., Optik 223, 165610 (2020).
S. F. Wang, X. T. Zu, G. Z. Sun, et al., Ceram. Int. 42, 19133 (2016).
S. F. Wang, Q. Li, X. T. Zu, et al., J. Magn. Magn. Mater. 419, 464 (2016).
Y. Zhang, J. Liu, G. Wu, and W. Chen, Nanoscale 4, 5300 (2012).
I. Omkaram and S. Buddhudu, Opt. Mater. 32, 8 (2009).
S. Wang, H. Gao, L. Fang, et al., Chem. Eng. J. Adv. 6, 100089 (2021).
A. Ullah, M. Usman, W. Qingyu, et al., Opt. Mater. 116, 111097 (2021).
S. Wang, H. Gao, J. Li, et al., J. Phys. Chem. Solid 150, 109891 (2021).
P. Singh, A. Sudhaik, P. Raizada, et al., Mater. Today Chem. 12, 85 (2019).
J. Li, S. Wang, G. Sun, et al., Mater. Today Chem. 19, 100390 (2021).
P. Prajapat, S. Dhaka, and H. S. Mund, J. Electron. Mater. 50, 4671 (2021).
M. Kaur, M. K. Ubhi, J. K. Grewal, and D. Singh, J. Phys. Chem. Solid 154, 110060 (2021).
A. Syed, A. H. Bahkali, and A. M. Elgorban, Opt. Mater. 113, 110595 (2021).
S. Wang, X. Chen, H. Gao, et al., J. Nano Res. 67, 1 (2021).
H. Gao, H. Yang, and S. Wang, Trans. Indian Ceram. Soc. 77, 150 (2018).
Y. Yang, Y. Jiang, Y. Wang, et al., Mater. Chem. Phys. 105, 154 (2007).
R. Dilip, R. Jayaprakash, P. Sangaiya, and S. Gopi, Result. Mater. 7, 100121 (2020).
S. Wang, S. Tang, H. Gao, et al., Opt. Mater. 118, 111273 (2021).
M. Shahid, L. **gling, Z. Ali, et al., Mater. Chem. Phys. 139, 566 (2013).
S. Taghavi Fardood, F. Moradnia, M. Mostafaei, et al., Nanochem. Res. 4, 86 (2019).
A. Becker, K. Kirchberg, and R. Marschall, Z. Phys. Chem. 234, 645 (2020).
B. Tan, Y. Fang, Q. Chen, et al., Opt. Mater. 109, 110470 (2020).
S. Mandizadeh, M. Salavati-Niasari, and M. Sadri, Sep. Purif. Technol. 175, 399 (2017).
ACKNOWLEDGMENTS
This work was supported by project of the energy bureau of Jilin province of China, grant no. 3d516l911425.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Chen, Y., Liu, M. Preparation and Study of the Adsorption Performances of (Mg,Ca,Ba)Fe2O4 Magnetic Porous Materials for Removal of Cd(II) Heavy Metal Ion from Water Environment. Russ. J. Phys. Chem. 96, 1761–1767 (2022). https://doi.org/10.1134/S0036024422080283
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422080283