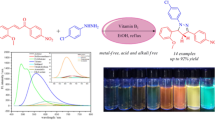
Abstract
Some fluorescent benzimidazole derivatives have been designed and synthesized using cobalt(ii) hydroxide as highly efficient catalyst. Synthesized compounds have been characterized by 1H and 13C-NMR and mass spectral analysis. The solvent effect on the absorption and fluorescence bands has been analyzed and supplemented by computational studies. Solvatochromic effects on the spectral position and profile of the stationary fluorescence spectra clearly indicate the charge transfer (CT) character of the emitting singlet states of all of the compounds studied in both polar and non-polar environments. The fluorescence decays for the benzimidazoles fit satisfactorily to a single exponential kinetics. HOMO and LUMO orbital pictures [DFT/B3LYP/6-31G(d,p)] evidence the existence of excited state intramolecular proton transfer (ESIPT) in benzimidazole derivatives containing a hydroxy group.
Similar content being viewed by others
References
E. M. Across, K. M. White, R. S. Moshrefzadeh and C. V. Francis, Azobeneimidazole compounds and polymers for nonlinear optics, Macromolecules, 1995, 28, 2526–2532.
G. A. Jeffrey, An Introduction to Hydrogen Bonding, Oxford University Press, Oxford, England, 1997.
L. Pauling, The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules, J. Am. Chem. Soc., 1931, 53, 1367.
G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond in Structural Chemistry and Biology, Oxford University Press, New York, 1999.
J. Jayabharathi, V. Thanikachalam and K. Jayamoorthy, Physicochemical studies of chemosensor imidazole derivatives: DFT based ESIPT process, Spectrochim. Acta, Part A, 2012, 89, 168–176.
J. Jayabharathi, V. Thanikachalam, M. Vennila and K. Jayamoorthy, Potential fluorescent chemosensor based on l-tryptophan derivative: DFT based ESIPT process, Spectrochim. Acta, Part A, 2012, 95, 446–451.
P. T. Chou, M. L. Martinez and J. H. Clements, Reversal of excitation behavior of proton-transfer vs. charge- transfer by dielectric perturbation of electronic state manifolds, J. Phys. Chem., 1993, 97, 2618–2622.
L. F. Campo, F. S. Rodembusch and V. Stefani, New fluorescent monomers and polymers displaying an intramolecular proton - transfer mechanism in the electronically excited state (ESIPT). IV. Synthesis of acryloylamide and diallylamino benzazole dyes and its copolymerization with MMA, J. Appl. Polym. Sci., 2006, 99, 2109–2116.
Y. Wu, X. Peng, J. Fan, S. Gao, M. Tian, J. Zhao and S. Sun, Fluorescence sensing of anions based on inhibition of excited-state intramolecular proton transfer, J. Org. Chem., 2007, 72, 62–70.
S. Park, O. H. Kwon, Y. S. Lee, D. J. Jang and S. Y. Park, Imidazole-based excited-state intramolecular proton-transfer (ESIPT) materials: observation of thermally activated delayed fluorescence (TDF), J. Phys. Chem. A, 2007, 111, 9649–9653.
O. K. Abou-Zied, R. Jimenez and F. E. Romesberg, Tautomerization dynamics of a model base-pair in DNA, J. Am. Chem. Soc., 2001, 123, 4613–4614.
W. S. Yu, C. C. Cheng, Y. M. Cheng, P. C. Wu, Y. H. Song, Y. Chi and P. T. Chou, Excited state intra molecular proton transfer in five membered hydrogen bonding system: 2-pyridyl pyrazoles, J. Am. Chem. Soc., 2003, 125, 10800–10801.
C. L. Chen, C. W. Lin, C. C. Hsieh, C. H. Lai, G. H. Lee, C. C. Wang and P. T. Chou, Dual excited-state intramolecular proton transfer reaction in 3-hydroxy-2-(pyridin-2-yl)-4H-chromen-4-one, J. Phys. Chem. A, 2009, 113, 205–214.
K. Y. Chen, C. C. Hsieh, Y. M. Cheng, C. H. Lai and P. T. Chou, Extensive spectral tuning of the proton transfer emission from 550 to 675 nm via a rational derivatization of 10-hydroxybenzo[h]quinoline, Chem. Commun., 2006, 4395–4397.
K. Y. Chen, Y. M. Cheng, C. H. Lai, C. C. Hsu, M. L. Ho, G. H. Lee and P. T. Chou, Ortho green protein synthetic chromophore; excited-state intramolecular proton transfer via a six membered ring hydrogen bonding system, J. Am. Chem. Soc., 2007, 129, 4534–4535.
E. M. Itskovitch, J. Ulstrup and M. A. Vorotyntsev, in The chemical physics of solvation part B, ed. R. R. Dogonadze, E. Kalman, A. A. Kornyshev and J. Ulstrup, Elsevier, Amsterdam, 1986.
A. Siemiarczuk, Z. R. Grabowski, A. Krówczyński, M. Asher and M. Ottolenghi, Two emitting states of excited p-(9-anthryl)-N,N-dimethylaniline derivatives in polar solvents, Chem. Phys. Lett., 1977, 51, 315–320.
A. Siemiarczuk and W. R. Ware, Complex excited state relaxation in p(9-Anthryl)-N,N dimethylaniline derivative evidenced by fluorescence lifetime distributions, J. Phys. Chem., 1987, 91, 3677–3682.
K. Tominaga, G. C. Walker, W. Jarzeüba and P. F. Barbara, Ultrafast charge separation in ADMA: experiment and theoretical issues, J. Phys. Chem., 1991, 95, 10475–10485.
K. Tominaga, G. C. Walker, T. J. Kang, P. F. Barbara and T. Fonseca, Reaction rates in the phenomenological adiabatic excited-state electron-transfer theory, J. Phys. Chem., 1991, 95, 10485–10492.
T. Okada, N. Mataga, W. Baumann and A. Siemiarczuk, Picosecond laser spectroscopy of 4-(9-anthryl-) N,N-dimethylaniline and related compounds, J. Phys. Chem., 1987, 91, 4490–4495.
A. Thiruvalluvar, S. Rosepriya, K. Jayamoorthy, J. Jayabharathi, S. O. Yildirim and R. J. Butcher, 2-(1-Phenyl-1H-benzimidazol-2-yl)phenol, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2013, 69, o62.
I. Lopez Arbeloa, Fluorescence quantum yield evaluation: corrections for re-absorption and re-emission, J. Photochem., 1980, 14, 97–105.
C. Reichardt, Empirical parameters of solvent polarity as linear free energy relationships, Angew. Chem., Int. Ed. Engl., 1979, 18, 98–110.
E. Lippert, Spektroskopische bistimmung des dipolmomentes aromatischer verbindugenim ersten angeregten singulettzustand, Z. Electrochem., 1957, 61, 962–975.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03 (Revision E.01), Gaussian, Inc., Wallingford, CT, 2004.
S. Dhar, D. K. Rana, S. S. Roy, S. Roy, S. Bhattacharya and S. C. Bhattacharya, Effect of solvent environment on the photophysics of a newly synthesized bioactive 7-oxy(5-selenocyanato-pentyl)-2H-1-benzopyran-2-one, J. Lumin., 2012, 132, 957–964.
R. A. Marcus, Free energy of non equilibrium polarization systems. II. Homogeneous and electrode systems, J. Chem. Phys., 1963, 38, 1858.
G. W. Castellan, Physical Chemistry, Narosa Publishing House, Delhi, 3rd edn, 1985.
M. J. Kamlet and R. W. Taft, The solvatochromic comparison method. 1. The scale of solvent hydrogen-bond acceptor (HBA) basicities, J. Am. Chem. Soc., 1976, 98, 377–383.
J. Catalan, V. Lopez and P. Perez, Use of the SPP scale for the analysis molecular system with dual emissions resulting from the solvent polarity, J. Fluoresc., 1996, 6, 15–22.
W. Rettig and M. Zander, On twisted intramolecular charge transfer (TICT) stated in N-aryl-carbazoles, Chem. Phys. Lett., 1982, 87, 229–234.
K. Chiba, J. I. Aihara, K. Araya and Y. Matsunaga, Bull. Chem. Soc. Jpn., 1980, 53, 1703.
L. Onsager, Electric moments of molecules in liquids, J. Am. Chem. Soc., 1936, 58, 1486–1493.
C. J. F. Böttcher, O. C. Van Belle, P. Bordewijk and A. Rip, in Theory of Electric Polarization, Elsevier, Amsterdam, 1973, vol. I.
E. Z. Lippert, Z. Naturforsch., A: Phys. Sci., 1955, 10, 541–546.
N. Mataga, Y. Kaifu and M. Koizumi, The solvent effect on fluorescence spectrum. Change of solute-solvent interaction during the lifetime of excited solute molecule, Bull. Chem. Soc. Jpn., 1955, 28, 690–691.
W. Liptay, in Excited States, ed. E. C. Lim, Academic Press, New York, 1974, p. 129.
E. G. McRae, Theory of solvent effects on molecular electronic spectra. Frequency shifts, J. Phys. Chem., 1957, 61, 562–572.
J. Jayabharathi, V. Thanikachalam, K. Jayamoorthy and N. Srinivasan, Synthesis, spectral studies and solvatochromism of some novel benzimidazole derivatives - ESIPT process, Spectrochim. Acta, Part A, 2013, 105, 223–228.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c3pp50083g
Rights and permissions
About this article
Cite this article
Jayabharathi, J., Thanikachalam, V. & Jayamoorthy, K. Synthesis of some fluorescent benzimidazole derivatives using cobalt(ii) hydroxide as highly efficient catalyst — spectral and physico-chemical studies and ESIPT process. Photochem Photobiol Sci 12, 1761–1773 (2013). https://doi.org/10.1039/c3pp50083g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c3pp50083g