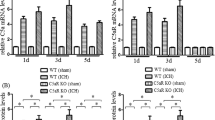
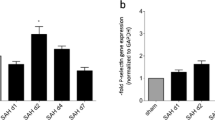
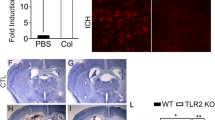
Abstract
Spontaneous intracerebral haemorrhage (ICH) is the most devastating stroke subtype and has no proven treatment. von Willebrand factor (VWF) has recently been demonstrated to promote inflammation processes. The present study investigated the pathophysiological role of VWF after experimental ICH. Functional outcomes, brain edema, blood-brain barrier (BBB) permeability, cerebral inflammation and levels of intercellular adhesion molecule-1 (ICAM-1) and matrix metalloproteinase-9 (MMP-9) were measured in a mouse model of ICH induced by autologous blood injection. We show that VWF were increased in the plasma and was accumulated in the perihematomal regions of mice subjected to ICH. Injection of VWF resulted in incerased expression of proinflammatory mediators and activation of ICAM-1 and MMP-9, associated with elevated myeloperoxidase, recruitment of neutrophils and microglia. Moreover, mice treated with VWF showed dramatically decreased pericyte coverage, more severe BBB damage and edema formation, and neuronal injury was increased compared with controls. In contrast, blocking antibodies against VWF reduced BBB damage and edema formation and improved neurological function. Together, these data identify a critical role for VWF in cerebral inflammation and BBB damage after ICH. The therapeutic interventions targeting VWF may be a novel strategy to reduce ICH-related injury.
Similar content being viewed by others
Introduction
von Willebrand factor (VWF) is an ultra-large multimeric glycoprotein, which is present in Weibel-Palade bodies of endothelial cells and alpha granules of platelets and is released in circulation upon activation1. VWF plays a crucial role in platelet adhesion and aggregation after vascular injury and under conditions of high shear stress2,3. Recently, VWF was also shown to mediate leukocyte extravasation and inflammatory response. Animal studies have shown that VWF deficiency reduced inflammatory cell recruitment, atherosclerotic lesion and ischemic cerebral injury4,5,6, and blocking antibody against VWF inhibited neutrophil extravasation in peritonitis and was protected from myocardial ischemic injury5,7.
Spontaneous intracerebral haemorrhage (ICH), defined as spontaneous bleeding from intraparenchymal blood vessels in the absence of trauma, represents roughly 10–15% of all stroke subtypesGelatin zymography Zymography was performed as described47. In brief, 40 μg protein samples were mixed with SDS sample buffer and loaded onto 10% Tris-Glycine gels containing 0.1% gelatin. After electrophoresis, gels were washed with 2.5% Triton X-100 for 30 minutes and incubated in develo** buffer (50 mM Tris pH 7.6, 0.2 mM NaCl, 5 mM CaCl2, 0.02% Brij-35) at 37 °C for 48 hours. Gels were stained with 0.5% Coomassie blue R-250 (Sigma-Aldrich) for 3 hour and destained appropriately. Gels were scanned and analyzed using Quantity One software (Bio-Rad). Twenty four hours after ICH induction, mice were euthanized by overdose of chloral hydrate, perfused transcardially with ice-cold PBS and 4% paraformaldehyde (pH 7.4; Sigma-Aldrich). The brains were removed and fixed in 4% paraformaldehyde overnight and then cryoprotected in 30% sucrose overnight at 4 °C. Frozen sections were cut in the coronal plane at 20 μm on a cryostat (Leica Microsystems Inc., Buffalo Grove, IL, USA). Immunohistochemistry was performed as described49,50. Images were obtained using an Olympus BX 51 microscope and an Olympus FV 1,000 confocal microscope. Colocalization was verified and reconstructed using Olympus FV 10-ASW software. Primary antibodies used were: rabbit anti-VVW (ab154193, Abcam), rabbit anti-human VWF (Dako), rat anti-CD31 (PECAM-1, BD Pharmingen, San Diego, CA, USA), rat anti-mouse LY-6B.2 (AbD Serotec, Raleigh, NC, USA), goat anti-Iba1 (Ionized calcium binding adaptor molecule 1, Abcam, Cambridge, MA, USA) and FITC-conjugated rat anti-mouse aminopeptidase N (CD13) (BD Pharmingen). Secondary antibodies used were: Alexa Fluor 488 donkey anti-rabbit immunoglobulin G (IgG), Alexa Fluor 594 donkey anti-rat IgG, Alexa Fluor 594 donkey anti-goat IgG, and Alexa Fluor 594 donkey anti-rabbit IgG (Invitrogen). Nuclei were stained with DAPI. For quantification of LY6B and Iba1 labeled cells in the perihematoma area, four fields from each section were captured under 40X objective. Each image was traced using ImageJ software. The total numbers of positive cells in the traced area were counted and expressed as per mm2. Pericyte coverage was determined as a percentage of CD13-positive area covering CD31-positive area in 0.42 mm2 regions. For each animal, 3 sections (400 μm apart) from the ipsilateral hemispheres were analyzed. Twenty four hours after ICH induction, mice were transcardially perfused and the brains were removed and separated into hemispheres ipsilateral and contralateral to haemorrhage. Ipsilateral hemispheres were cut into 4 mm thickness block around the needle track. The brain tissues were homogenized in RIPA lysis buffer (Millipore) containing protease inhibitor cocktails (Roche Diagnostics GmbH, Mannheim, Germany). Equal amounts of protein samples were loaded on 10% SDS-PAGE gel, electrophoresed, and transferred onto PVDF membranes. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween 20 and incubated with goat anti-mouse ICAM-1 (R&D systems), and rabbit anti-β-actin (Cell Signaling Technology) antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Signals were detected with an enhanced chemoluminescence solution (Millipore) and quantified by scanning densitometry using a Bio-Image Analysis System (Bio-Rad). Mice were injected with 2% Evans blue in PBS (4 ml/kg; Sigma-Aldrich) at 21 hours after ICH induction, followed 3 hours later by transcardiac perfusion. The hemorrhagic brain hemispheres were removed and placed in formamide (Sigma-Aldrich) for 72 hours. The amount of extravasated Evans blue dye was evaluated at 620 nm49. Neurological deficits were assessed by an investigator blinded to the treatment of the animals at 1 and 3 days after ICH. For the quantification of neurological deficits, a 5 point neurological score was employed: 0, no neurological deficit; 1, forelimb weakness; 2, spontaneous circling; 3, partial paralysis on one side; 4, absence of spontaneous movement or unconsciousness; 5, death. Data are represented as means ± standard errors of the means. Statistical analysis were performed using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. Differences were determined by using the Student two-tailed t test when two groups were compared. Behavior data were compared using Mann-Whitney U test. P values less than 0.05 were considered as statistically significant.Immunofluorescence
Western blotting
BBB permeability
Neurobehavioral scores
Statistics
Additional Information
How to cite this article: Zhu, X. et al. von Willebrand factor contributes to poor outcome in a mouse model of intracerebral haemorrhage. Sci. Rep. 6, 35901; doi: 10.1038/srep35901 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Vischer, U. M. & de Moerloose, P. von Willebrand factor: from cell biology to the clinical management of von Willebrand’s disease. Crit. Rev. Oncol. Hematol. 30, 93–109 (1999).
Nieswandt, B. & Stoll, G. The smaller, the better: VWF in stroke. Blood. 115, 1477–1478 (2010).
Ruggeri, Z. M. Von Willebrand factor, platelets and endothelial cell interactions. J. Thromb. Haemost. 1, 1335–1342 (2003).
Chauhan, A. K. et al. ADAMTS13: a new link between thrombosis and inflammation. J. Exp. Med. 205, 2065–2074 (2008).
Gandhi, C., Khan, M. M., Lentz, S. R. & Chauhan, A. K. ADAMTS13 reduces vascular inflammation and the development of early atherosclerosis in mice. Blood. 119, 2385–2391 (2012).
Zhao, B. Q. et al. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 114, 3329–3334 (2009).
Petri, B. et al. von Willebrand factor promotes leukocyte extravasation. Blood. 116, 4712–4719 (2010).
Keep, R. F., Hua, Y. & **, G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet. Neurol. 11, 720–731 (2012).
Ziai, W. C. Hematology and inflammatory signaling of intracerebral hemorrhage. Stroke. 44, S74–S78 (2013).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 468, 562–566 (2010).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat Neurosci. 14, 1398–1405 (2011).
Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 508, 55–60 (2014).
Imai, T. et al. Nrf2 activator ameliorates hemorrhagic transformation in focal cerebral ischemia under warfarin anticoagulation. Neurobiol. Dis. 89, 136–146 (2016).
Fernandez-Klett, F. et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood. Flow. Metab. 33, 428–439 (2013).
Bell, R. D. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 485, 512–516 (2012).
Suidan, G. L. et al. Endothelial Von Willebrand factor promotes blood-brain barrier flexibility and provides protection from hypoxia and seizures in mice. Arterioscler. Thromb. Vasc. Biol. 33, 2112–2120 (2013).
Brill, A. et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 117, 1400–1407 (2011).
Hillgruber, C. et al. Blocking von Willebrand factor for treatment of cutaneous inflammation. J. Invest. Dermatol. 134, 77–86 (2014).
Noubade, R. et al. von-Willebrand factor influences blood brain barrier permeability and brain inflammation in experimental allergic encephalomyelitis. AM. J. Pathol. 173, 892–900 (2008).
Zhao, X., Zhang, Y., Strong, R., Grotta, J. C. & Aronowski, J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J. Cereb. Blood. Flow. Metab. 26, 811–820 (2006).
Rosenberg, G. A. & Navratil, M. Metalloproteinase inhibition blocks edema in intracerebral hemorrhage in the rat. Neurology. 48, 921–926 (1997).
del Zoppo, G. J. Acute anti-inflammatory approaches to ischemic stroke. Ann. N. Y. Acad. Sci. 1207, 143–148 (2010).
Fan, X., Lo, E. H. & Wang, X. Effects of minocycline plus tissue plasminogen activator combination therapy after focal embolic stroke in type 1 diabetic rats. Stroke. 44, 745–752 (2013).
Kleinschnitz, C. et al. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 115, 2323–2330 (2007).
**, G., Strahle, J., Hua, Y. & Keep, R. F. Progress in translational research on intracerebral hemorrhage: is there an end in sight? Prog. Neurobiol. 115, 45–63 (2014).
Bernardo, A. et al. Platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings support leukocyte tethering and rolling under high shear stress. J. Thromb. Haemost. 3, 562–570 (2005).
Andre, P. et al. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood. 96, 3322–3328 (2000).
Dole, V. S., Bergmeier, W., Mitchell, H. A., Eichenberger, S. C. & Wagner, D. D. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood. 106, 2334–2339 (2005).
Bath, P. M., Blann, A., Smith, N. & Butterworth, R. J. Von Willebrand factor, P-selectin and fibrinogen levels in patients with acute ischaemic and haemorrhagic stroke, and their relationship with stroke sub-type and functional outcome. Platelets. 9, 155–159 (1998).
Vergouwen, M. D. et al. Reduced ADAMTS13 activity in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Cereb. Blood. Flow. Metab. 29, 1734–1741 (2009).
Gandhi, C., Motto, D. G., Jensen, M., Lentz, S. R. & Chauhan, A. K. ADAMTS13 deficiency exacerbates VWF-dependent acute myocardial ischemia/reperfusion injury in mice. Blood. 120, 5224–5230 (2012).
Zhang, L. et al. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 112, 3486–3494 (2005).
Ma, Q. et al. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J. Cereb. Blood. Flow. Metab. 31, 881–893 (2011).
Pfefferkorn, T. & Rosenberg, G. A. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 34, 2025–2030 (2003).
Amenta, P. S., Jallo, J. I., Tuma, R. F., Hooper, D. C. & Elliott, M. B. Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J. Neuroinflammation. 11, 191 (2014).
Claus, R. A., Bockmeyer, C. L., Sossdorf, M. & Lösche, W. The balance between von-Willebrand factor and its cleaving protease ADAMTS13: biomarker in systemic inflammation and development of organ failure? Curr. Mol. Med. 10, 236–248 (2010).
Tsubokawa, T. et al. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 37, 1888–1894 (2006).
del Zoppo, G. J. Acute anti-inflammatory approaches to ischemic stroke. Ann. N. Y. Acad. Sci. 1207, 143–148 (2010).
Yang, Y. & Rosenberg, G. A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 42, 3323–3328 (2011).
Rynkowski, M. A. et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat. Protoc. 3, 122–128 (2008).
Glascock, J. J. et al. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J. Vis. Exp. 56, e2968 (2011).
Snyder, E. Y., Taylor, R. M. & Wolfe, J. H. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 374, 367–370 (1995).
Passini, M. A. & Wolfe, J. H. Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J. Virol. 24, 12382–12392 (2001).
Yepes, M. et al. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 112, 1533–1540 (2003).
Su, E. J. et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat. Med. 14, 731–737 (2008).
Lenting, P. J. et al. An experimental model to study the in vivo survival of von Willebrand factor. Basic aspects and application to the R1205H mutation. J.Biol. Chem. 279, 12102–12109 (2004).
Cai, P. et al. Recombinant ADAMTS 13 Attenuates Brain Injury After Intracerebral Hemorrhage. Stroke. 46, 2647–2653 (2015).
Fan, W. et al. Caspase-3 modulates regenerative response after stroke. Stem cells 32, 473–486 (2014).
Wang, L. et al. Recombinant ADAMTS13 reduces tissue plasminogen activator-induced hemorrhage after stroke in mice. Ann. Neurol. 73, 189–198 (2013).
Zhao, B. Q. et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med. 12, 441–445 (2006).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Key Program 81530034, General Program 30971014 and 81071062 to B.Q.Z., General Program 81271302 and 81070914 to J.R.L., General Program 81471331 to W.F.), the Natural Science Foundation of Shanghai (14ZR1401800 to W.F.), the Research Innovation Project from Shanghai Municipal Science and Technology Commission (14JC1404300 to J.R.L.), the Open Fund of State Key Laboratory of Medical Neurobiology, Fudan University (SKLMN2014001 to J.R.L.), and the Project from SHSMU-ION Research Center for Brain Disorders (2015 to J.R.L.).
Author information
Authors and Affiliations
Contributions
X.Z., Y.C., L.W., P.C., H.X., H.L., L.L., X.B. and W.F. performed experiments. X.Z., Y.C., L.W., P.C., H.X., J.R.L. and W.F. analyzed data. X.Z., W.F., J.R.L. and B.Q.Z. designed and interpreted experiments. X.Z., W.F., J.R.L. and B.Q.Z. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhu, X., Cao, Y., Wei, L. et al. von Willebrand factor contributes to poor outcome in a mouse model of intracerebral haemorrhage. Sci Rep 6, 35901 (2016). https://doi.org/10.1038/srep35901
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep35901
- Springer Nature Limited
This article is cited by
-
Von Willebrand Factor Activity in Rats after Transient Cerebral Ischemia
Neuroscience and Behavioral Physiology (2021)
-
STO: Stroke Ontology for Accelerating Translational Stroke Research
Neurology and Therapy (2021)
-
Cancer cell-derived von Willebrand factor enhanced metastasis of gastric adenocarcinoma
Oncogenesis (2018)
-
Role of the Golgi Apparatus in the Blood-Brain Barrier: Golgi Protection May Be a Targeted Therapy for Neurological Diseases
Molecular Neurobiology (2018)