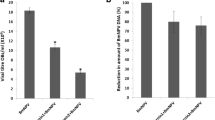
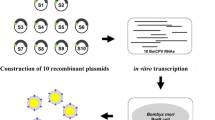
Abstract
Atlastin is a member of the dynamin protein superfamily and it can mediate homotypic fusion of endoplasmic reticulum (ER) membranes, which is required for many biological processes. In this study, a new Atlastin homologous protein, BmAtlastin-n, was characterized in silkworms and was found to contain an N-terminal conserved GTPase domain and a coiled-coil middle domain. BmAtlastin-n is localized in the cytoplasm and enriched in silkworm midgut. Results also showed that overexpression of BmAtlastin-n in BmN-SWU1 cells could enhance resistance to BmNPV. To better confirm its antiviral effect, microRNA was used to knock down the expression of BmAtlastin-n in BmE-SWU1 cells with inducing the reproduction of BmNPV. A transgenic expression vector of BmAtlastin-n was constructed and introduced to silkworm embryos by microinjection. The transgenic silkworm also showed considerable antiviral capacity. In conclusion, these findings demonstrate that BmAtlastin-n plays an important role in BmNPV defense. More importantly, the current study may provide a new clue for Atlastin research.
Similar content being viewed by others
Introduction
Atlastins belong to the dynamin superfamily, which is involved in several physiological functions of endomembrane in eukaryotic cells1, including formation of transport vesicles2, mitochondrial fusion and fission3, membrane fusion4 and pathogen resistance5. Nearly a decade ago, genetic mutation of Atlastin was identified as a cause of a form of autosomal dominant hereditary spastic paraplegia (HSP), which is characterized by progressive lower-extremity weakness and spasticity6. Atlastin has an N-terminal conserved GTPase domain, a three-helix-bundle (3HB) middle domain, two tandem transmembrane (TM) segments and a C-terminal cytoplasmic tail (CT)7. Current studies have reported that Atlastin is required for the formation and maintenance of ER networks8 and mediates the homotypic ER fusion and vesicle trafficking in the ER/Golgi interface9. In Drosophila melanogaster, overexpression of Atlastin causes expansion of the ER membranes and inhibition of Atlastin results in ER fragmentation10. The depletion of Atlastin2/3 can cause unbranched ER tubules in Hela cells8; in Xenopus egg extracts, Atlastin antibodies inhibit formation of the ER network11. Atlastin-1 is highly expressed in the brain and is enriched in growth cones, also benefits axon elongation during neuronal development12,13. Danio rerio Atlastin also controls larval mobility and spinal motor axon growth by inhibiting the bone morphogenetic protein (BMP) pathway14.
Atlastin has been found widely in many different species. Some of them, such as Homo sapiens, Xenopus laevis and Danio rerio, have three Atlastin isoforms. In others, such as Drosophila melanogaster and Caenorhabditis elegans, only one is present15. Bombyx mori, the silkworm, has four known Atlastin homeotic genes16. Silkworms are an important lepidopteran insect and its silk is an essential biological material in both textiles and medicine17,18,Preparation of BmAtlastin-n antibody (anti-BmAtlastin-n) The primers (forward 5′ cgggatccAGTCTCGGTGTGAAGCCAAAGG 3′ and reverse 5′ cggaattcCGCCTTGTTGTGGAGTGAATC 3′) were used to amplify fragments from vector pMD19-BmAtlastin-n and then constructed with pET32a-c (+) (Novagen). Vector pET32a-BmAtlastin-n was transformed into BL21 and induced by 0.5 mM IPTG at 37 °C for 5 h. The recombinant protein was purified by Ni affinity chromatography and the products were used for the immunization of New Zealand white rabbits (0.4 mg/each). Immune treatments were performed once per week for a total of four times and then the serum was collected to prepare antibody of BmAtlastin-n. Cells were lysed with lysis buffer (Beyotime, China) followed by washing twice with PBS and denatured at 100 °C with 5 × SDS-PAGE Loading Buffer (Beyotime). Proteins were subjected to 12% SDS–PAGE and the bands were transferred to PVDF membranes. They were then incubated with primary antibody anti-BmAtlastin-n/anti-VP39/anti-α-Tubulin (Beyotime) (1/5000, 1 h, 25 °C) and secondary antibody HRP conjugated anti-rabbit IgG/HRP conjugated anti-mouse IgG (Beyotime). The final results were analyzed with ECL Western Blotting Detection System (Bio-Rad). Cells were fixed with 4% paraformaldehyde for 15 min at 25 °C and permeated in 0.5% Triton X-100 (10 min, 25 °C), then blocked with Immunol Staining Blocking Buffer (Beyotime, 1 h, 37 °C). Cells were incubated with anti-BmAtlastin-n primary antibody (1:200, 1 h, 37 °C). Alexa Fluor 555 conjugated donkey anti-rabbit polyclonal (Beyotime) was used as the secondary antibody. Then the cells were imaged under a laser scanning confocal microscope (Olympus). The transgenic vector piggyBac [3 × P3-EGFP, IE1P-BmAtlastin-n] and the helper vector pHA3PIG were co-injected into the silkworm eggs within 4 h of spawning using a microinjector (Eppendorf)37,38. Then the G0 silkworms were reared at 25 °C. The G1 silkworms were produced by inbreeding and backcrosses of G0. BmAtlastin-n-OE transgenic silkworms were selected in G1 broods using a fluorescent stereomicroscope (Olympus), which were found to be EGFP positive in their compound eyes. The genomic DNA was extracted from BmAtlastin-n-OE transgenic silkworm and digested with Hae III for 16 h at 37 °C, then self-ligated in solution I (Takara). The products were used for performing the inverse PCR with the primers pBacL (forward 5′ ATCAGTGACACTTACCGCATTGACA 3′ and reverse 5′ TGACGAGCTTGTTGGTGAGGATTCT 3′) and pBacR (forward 5′ TACGCATGATTATCTTTAACGTA 3′ and reverse 5′ GTACTGTCATCTGATGTACCAGG 3′)34 and the fragments were cloned into pMD19-T vector (Takara) and sequenced. The results were analyzed in silkDB (http://www.silkdb.org/silkdb/). Fresh mulberry leaves were cut into circles 1 cm in diameter, then each piece was smeared with 1 × 106 ODVs. First-day fourth-instar larvae of BmAtlastin-n-OE transgenic silkworm and Dazao control silkworm were fed mulberry leaves with ODVs (one piece/larva). The silkworms, which each ate a whole piece of mulberry leaf, were collected for assessment of the mortality rate. Then a capillary tube was used to inject 1 × 106 BV particles/larva in the two lines form valve. Each treatment was performed in triplicate and each replicate included 100 larvae. The student’s t-test was used to assess any statistically significant differences between treatments. P-value < 0.05 was considered significant, here indicated with “*” and P-value < 0.01 was considered very significant, here indicated with “**”. Data from three independent experiments are here presented as means ± SEM.Western blotting
Immunofluorescence
Microinjection and screening
Analysis of the insertion site
Oral inoculation and injection of BmNPV
Statistical analysis
Additional Information
How to cite this article: Liu, T.- et al. A newly discovered member of the Atlastin family, BmAtlastin-n, has an antiviral effect against BmNPV in Bombyx mori. Sci. Rep. 6, 28946; doi: 10.1038/srep28946 (2016).
References
Moss, T. J., Daga, A. & McNew, J. A. Fusing a lasting relationship between ER tubules. Trends Cell Biol 21, 416–423 (2011).
Oh, P., McIntosh, D. P. & Schnitzer, J. E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol 141, 101–114 (1998).
Hoppins, S., Lackner, L. & Nunnari, J. The machines that divide and fuse mitochondria. Annu Rev Biochem 76, 751–780 (2007).
McNew, J. A., Sondermann, H., Lee, T., Stern, M. & Brandizzi, F. GTP-Dependent Membrane Fusion. Annu Rev Cell Dev Bi 29, 529–550 (2013).
MacMicking, J. D. IFN-inducible GTPases and immunity to intracellular pathogens. Trends Immunol 25, 601–609 (2004).
Zhao, X. P. et al. Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nat Genet 29, 326–331 (2001).
Wu, F. Y., Hu, X. Y., Bian, X., Liu, X. Q. & Hu, J. J. Comparison of human and Drosophila atlastin GTPases. Protein Cell 6, 139–146 (2015).
Hu, J. J. et al. A Class of Dynamin-like GTPases Involved in the Generation of the Tubular ER Network. Cell 138, 549–561 (2009).
Namekawa, M. et al. Mutations in the SPG3A gene encoding the GTPase atlastin interfere with vesicle trafficking in the ER/Golgi interface and Golgi morphogenesis. Mol Cell Neurosci 35, 1–13 (2007).
Orso, G. et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase Atlastin. Nature 460, 978–U958 (2009).
Liu, T. Y. et al. Lipid interaction of the C terminus and association of the transmembrane segments facilitate atlastin-mediated homotypic endoplasmic reticulum fusion. P Natl Acad Sci USA 109, E2146–E2154 (2012).
Zhu, P. P. et al. Cellular localization, oligomerization and membrane association of the hereditary spastic paraplegia 3A (SPG3A) protein atlastin. J Biol Chem 278, 49063–49071 (2003).
Zhu, P. P., Soderblom, C., Tao-Cheng, J. H., Stadler, J. & Blackstone, C. SPG3A protein atlastin-1 is enriched in growth cones and promotes axon elongation during neuronal development. Hum Mol Genet 15, 1343–1353 (2006).
Fassier, C. et al. Zebrafish atlastin controls motility and spinal motor axon architecture via inhibition of the BMP pathway. Nat Neurosci 13, 1380–U1112 (2010).
Rismanchi, N., Soderblom, C., Stadler, J., Zhu, P. P. & Blackstone, C. Atlastin GTPases are required for Golgi apparatus and ER morphogenesis. Hum Mol Genet 17, 1591–1604 (2008).
Chen, Q. M. et al. Identification and Expression Patterns of Atlastin Genes in Silkworm, Bombyx mori. Science of Sericulture 37, 0206–0214 (2011).
Altman, G. H. et al. Silk-based biomaterials. Biomaterials 24, 401–416 (2003).
MacIntosh, A. C., Kearns, V. R., Crawford, A. & Hatton, P. V. Skeletal tissue engineering using silk biomaterials. J Tissue Eng Regen M 2, 71–80 (2008).
**a, Q. Y. et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 306, 1937–1940 (2004).
Dong, X. L. et al. BmREEPa Is a Novel Gene that Facilitates BmNPV Entry into Silkworm Cells. Plos One 10, 0144575 (2015).
Pan, M. H. et al. Establishment and characterization of an ovarian cell line of the silkworm, Bombyx mori. Tissue Cell 42, 42–46 (2010).
Zhang, J. et al. Differential Susceptibilities to BmNPV Infection of Two Cell Lines Derived from the Same Silkworm Ovarian Tissues. Plos One 9, 0105986 (2014).
Keddie, B. A., Aponte, G. W. & Volkman, L. E. The Pathway Of Infection Of Autographa-Californica Nuclear Polyhedrosis-Virus In an Insect Host. Science 243, 1728–1730 (1989).
Ikeda, M., Hamajima, R. & Kobayashi, M. Baculoviruses: diversity, evolution and manipulation of insects. Entomol Sci 18, 1–20 (2015).
Li, G., Zhang, J. Y., Sun, Y., Wang, H. & Wang, Y. Q. The Evolutionarily Dynamic IFN-Inducible GTPase Proteins Play Conserved Immune Functions in Vertebrates and Cephalochordates. Mol Biol Evol 26, 1619–1630 (2009).
Al-Zeer, M. A., Al-Younes, H. M., Lauster, D., Abu Lubad, M. & Meyer, T. F. Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNG- inducible guanylate binding proteins. Autophagy 9, 50–62 (2013).
Anderson, S. L., Carton, J. M., Lou, J., **ng, L. & Rubin, B. Y. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology 256, 8–14 (1999).
Zhu, Z. X. et al. Nonstructural Protein 1 of Influenza A Virus Interacts with Human Guanylate-Binding Protein 1 to Antagonize Antiviral Activity. Plos One 8, 0055920 (2013).
Liu, Z. et al. The immunity-related GTPase Irgm3 relieves endoplasmic reticulum stress response during coxsackievirus B3 infection via a PI3K/Akt dependent pathway. Cell Microbiol 14, 133–146 (2012).
Itsui, Y. et al. Antiviral Effects of the Interferon-Induced Protein Guanylate Binding Protein 1 and Its Interaction with the Hepatitis C Virus NS5B Protein. Hepatology 50, 1727–1737 (2009).
Pan, W., Zuo, X. Y., Feng, T. T., Shi, X. H. & Dai, J. F. Guanylate-binding protein 1 participates in cellular antiviral response to dengue virus. Virol J 9 (2012).
Degrandi, D. et al. Murine Guanylate Binding Protein 2 (mGBP2) controls Toxoplasma gondii replication. P Natl Acad Sci USA 110, 294–299 (2013).
Jiang, L. & **a, Q. Y. The progress and future of enhancing antiviral capacity by transgenic technology in the silkworm Bombyx mori. Insect Biochem Molec 48, 1–7 (2014).
Jiang, L. et al. Resistance to BmNPV via Overexpression of an Exogenous Gene Controlled by an Inducible Promoter and Enhancer in Transgenic Silkworm, Bombyx mori. Plos One 7, 0041838 (2012).
Jiang, L. et al. Resistance to Bombyx mori nucleopolyhedrovirus via overexpression of an endogenous antiviral gene in transgenic silkworms. Arch Virol 157, 1323–1328 (2012).
Zhang, J. et al. Inhibition of BmNPV replication in silkworm cells using inducible and regulated artificial microRNA precursors targeting the essential viral gene lef-11. Antivir Res 104, 143–152 (2014).
Tamura, T. et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol 18, 559–559 (2000).
Thomas, J. L., Da Rocha, M., Besse, A., Mauchamp, B. & Chavancy, G. 3xP3-EGFP marker facilitates screening for transgenic silkworm Bombyx mori L. from the embryonic stage onwards. Insect Biochem Molec 32, 247–253 (2002).
Acknowledgements
We greatly thank Dr. Wei Wang and Dr. Ding-pei Long for the supports for this experiments. This work was supported by the National Natural Science Foundation of China (Grant No. 31472152, 31272505 and 31572466) China Postdoctoral Science Foundation (2015M582502), Chongqing Postdoctoral Science Foundation (Xm2015121) and China Agriculture Research System (CARS-22).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: T.-h.L., X.-l.D., C.-x.P. and M.-h.P. Experiments performance: T.-h.L., X.-l.D., C.-x.P., G.-y.D. and Y.-f.W. Data analysis: T.-h.L., C.-x.P. and J.-g.Y. Reagents/materials/analysis tools contribution: P.C., C.L. and M.-h.P. Paper written: T.-h.L., X.-l.D. and M.-h.P.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, Th., Dong, Xl., Pan, Cx. et al. A newly discovered member of the Atlastin family, BmAtlastin-n, has an antiviral effect against BmNPV in Bombyx mori. Sci Rep 6, 28946 (2016). https://doi.org/10.1038/srep28946
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28946
- Springer Nature Limited