Abstract
Antibody-drug conjugates (ADCs) are a class of immunotherapeutic agents that enable the delivery of cytotoxic drugs to target malignant cells. Because various cancers and tumour vascular endothelia strongly express anti-human tissue factor (TF), we prepared ADCs consisting of a TF-specific monoclonal antibody (mAb) linked to the anticancer agent (ACA) monomethyl auristatin E (MMAE) via a valine-citrulline (Val-Cit) linker (human TF ADC). Identifying the most efficient drug design in advance is difficult because ADCs have complicated structures. The best method of assessing ADCs is to examine their selectivity and efficiency in releasing and distributing the ACA within tumour tissue. Matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS) can be used to directly detect the distributions of native molecules within tumour tissues. Here, MALDI-IMS enabled the identification of the intratumour distribution of MMAE released from the ADC. In conclusion, MALDI-IMS is a useful tool to assess ADCs and facilitate the optimization of ADC design.
Similar content being viewed by others
Introduction
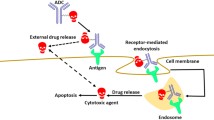
Antibody-drug conjugates (ADCs) are a class of immunotherapeutic agents that enable the delivery of cytotoxic drugs to target malignant cells. ADCs passively accumulate in solid tumour tissue through the enhanced permeability and retention effect1 and actively accumulate in target malignant cells because of the specific antibody-antigen binding2. The selection of a suitable monoclonal antibody (mAb), an anticancer agent (ACA), and a linker have supported the clinical success of ADCs3. The ADC strategy should be confined to highly toxic ACAs and not applied to ordinary ACAs, such as taxane, adriamycin, and others, because fewer than four ACA molecules should be conjugated to the mAb to prevent a decrease in the affinity of the mAb when too many ACA molecules are attached4. Monomethyl auristatin E (MMAE) is one of the most useful and potent ACAs for the clinical development of novel ADCs5,6. MMAE inhibits cell division by blocking the polymerization of tubulin.
In our laboratory, we have created an anti-human tissue factor (TF) mAb attached to valine-citrulline (Val-Cit)-MMAE (human TF ADC) and have reported its antitumour effect against xenografts of a human pancreatic cancer cell line, BxPC-37. The Val-Cit-MMAE has been designed for maximum serum stability and efficient release into the tumour environment8. Once human TF ADC binds to the target malignant cells, it is internalized by endocytosis, and MMAE is theoretically released into the tumour environment through the action of the lysosomal enzymes on the linker. TF is a transmembrane glycoprotein involved in the initiation of the extrinsic pathway of blood coagulation9, is expressed in various types of cancer, and plays a role in cancer progression, angiogenesis, tumour growth, and metastasis10. Because pancreatic cancer tissue expresses high levels of TF, it is a useful target antigen for this condition11,12,13.
To optimize the efficacy of an ADC against TF-positive solid tumours, a preclinical pharmacological evaluation of the ADC should be performed to determine whether the human TF ADC has been optimally designed. Regarding antitumour effects, ACAs must penetrate the tumour tissue efficiently and be retained there at a high and biologically active concentration14,15. For such analyses, high-performance liquid chromatography (HPLC) or liquid chromatography mass spectrometry (LC-MS) is generally used. However, these techniques do not provide information about the drug distribution in a specific target area, although they allow optimization of the drug design to a certain extent, enabling more efficiently targeted delivery. Autoradiography can be used to examine the tissue distribution of radiolabelled small molecules16. However, this method cannot distinguish between a radiolabelled drug conjugated to an ADC and free radiolabelled drug released from the ADCs23.
LC-MS/MS
The samples were analysed using a liquid chromatograph tandem mass spectrometer API3200 LC-MS/MS system (AB SCIEX, Framingham, MA). The analytical column, a reversed-phase LC column (4.0 μm polar, 80 Å; 50 × 3.0 mm, Synergi; Shimazu), was heated to 40 °C. The injection volume was 10 μm, and the flow rate was 0.4 mL/min. The autosampler was equipped with a cooling stack set at 4 °C. Acetonitrile and a 0.1% (w/v) formic acid solution were used as the mobile phases. For the gradient elution, the mobile phase composition was as follows: 5% acetonitrile for 1.0 min, increased to 40% for 2.0 min, increased to 100% for 2.0 min, maintained at 100% for 2.0 min, and then decreased back to 5.0% for 2.0 min. The mobile phase was introduced into the spectrometer via electrospray ionization in positive-ion mode under multiple reaction monitoring (MRM) conditions. The MRM transitions were used for MMAE (m/z 718.4/152.2) and MMAF as an internal standard (m/z 732.4/170.3). The standard curve had a linear range from 0.10 nM to 100 nM. Samples from the non-treated BxPC-3 tumour tissues were used as a negative control.
Immunohistochemistry
The 10-μm frozen sections were fixed with 4% paraformaldehyde (Wako) for 10 min. To block endogenous peroxidase, 3% hydrogen peroxide for 20 min was used. After being blocked with 3% skim milk in PBS for 30 min, the sections were incubated with an anti-rat IgG goat antibody (Histostar; MBL, Nagoya, Japan) for mAb/ADC according to the manufacturer’s instructions. The continuous sections were incubated with an anti-mouse CD31 goat antibody for vascular endothelial cells (10 μg/mL; R&D Systems, Minneapolis, MN) for 1 h at room temperature. After being washed with PBS, the sections were incubated with a horseradish peroxidase (HRP)-conjugated anti-goat IgG antibody (Jackson IR, West Grove, PA) according to the manufacturer’s instructions. Counterstaining was performed using haematoxylin. Negative controls included the replacement of the primary antibody by PBS and isotype antibodies.
Statistics
Measured differences were considered significant at P < 0.05. For the quantification of MMAE using LC-MS/MS, statistical significance was determined using the Tukey-Kramer multiple comparison test. To distinguish between MMAE alone and MMAE conjugated to a mAb, statistical significance was determined using the Tukey-Kramer multiple comparison test. For the high-resolution MALDI-IMS of MMAE in tumour tissues, statistical significance was determined using the two-sided Student’s t-test. The bar graph data were expressed as the mean ± standard deviation (SD). The error bars indicated the SD. The statistical analyses were performed using Statcal QC (The publisher OMS, Saitama, Japan).
Additional Information
How to cite this article: Fujiwara, Y. et al. Imaging mass spectrometry for the precise design of antibody-drug conjugates. Sci. Rep. 6, 24954; doi: 10.1038/srep24954 (2016).
References
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46, 6387–92 (1986).
Hellstrom, K. E., Hellstrom, I. & Brown, J. P. Human tumor-associated antigens identified by monoclonal antibodies. Springer Semin Immunopathol 5, 127–46 (1982).
Sievers, E. L. & Senter, P. D. Antibody-drug conjugates in cancer therapy. Annu Rev Med 64, 15–29 (2013).
Junutula, J. R. et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 26, 925–32 (2008).
Thudium, K. et al. American Association of Pharmaceutical Scientists National Biotechnology Conference Short Course: Translational Challenges in Develo** Antibody-Drug Conjugates: May 24, 2012, San Diego, CA. MAbs 5, 5–12 (2013).
Zolot, R. S., Basu, S. & Million, R. P. Antibody-drug conjugates. Nat Rev Drug Discov 12, 259–60 (2013).
Koga, Y. et al. Antitumor effect of anti-tissue factor antibody-MMAE conjugate in human pancreatic tumor xenografts. Int J Cancer 137, 1257–1466 (2015).
Sanderson, R. J. et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res 11, 843–52 (2005).
Chu, A. J. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam 2011, 367284 (2011).
van den Berg, Y. W., Osanto, S., Reitsma, P. H. & Versteeg, H. H. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood 119, 924–32 (2012).
Flossel, C., Luther, T., Muller, M., Albrecht, S. & Kasper, M. Immunohistochemical detection of tissue factor (TF) on paraffin sections of routinely fixed human tissue. Histochemistry 101, 449–53 (1994).
Nitori, N. et al. Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin Cancer Res 11, 2531–9 (2005).
Khorana, A. A. et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res 13, 2870–5 (2007).
Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nat Rev Cancer 6, 583–92 (2006).
Kim, M., Gillies, R. J. & Rejniak, K. A. Current advances in mathematical modeling of anti-cancer drug penetration into tumor tissues. Front Oncol 3, 278 (2013).
Solon, E. G. Use of radioactive compounds and autoradiography to determine drug tissue distribution. Chem Res Toxicol 25, 543–55 (2012).
**e, H., Audette, C., Hoffee, M., Lambert, J. M. & Blattler, W. A. Pharmacokinetics and biodistribution of the antitumor immunoconjugate, cantuzumab mertansine (huC242-DM1), and its two components in mice. J Pharmacol Exp Ther 308, 1073–82 (2004).
Mainini, V. et al. Detection of high molecular weight proteins by MALDI imaging mass spectrometry. Mol Biosyst 9, 1101–7 (2013).
Yasunaga, M. et al. The significance of microscopic mass spectrometry with high resolution in the visualisation of drug distribution. Sci Rep 3, 3050 (2013).
Stacy Shifflett Shord, S. J. S., Hong Zhao, Brian Booth, Nam Atiqur Rahman. Regulatory considerations for clinical pharmacology during development of antibody-drug conjugates. J Clin Oncol 33, 2015 (suppl; abstr 2569) (2015).
Furuichi, Y. et al. Imaging mass spectrometry reveals fiber-specific distribution of acetylcarnitine and contraction-induced carnitine dynamics in rat skeletal muscles. Biochim Biophys Acta 1837, 1699–1706 (2014).
Aichler, M. & Walch, A. MALDI Imaging mass spectrometry: current frontiers and perspectives in pathology research and practice. Lab Invest 95, 422–31 (2015).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–5 (2012).
Acknowledgements
We thank S. Saijou, F. Furuya, and S. Hanaoka for assistance in producing the anti-human TF mAb. We also thank Mrs. M. Nakayama for her secretarial support. This work was supported by the National Cancer Center Research and Development Fund (26-A-14 for Y.M., 26-A-12 for M.Y.); the Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour, and Welfare of Japan (Y.M.); a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, (Y.M.); and Practical Research for Innovative Cancer Control (15ck0106114h0002) from the Japan Agency for Medical Research and Development, AMED (M.Y.).
Author information
Authors and Affiliations
Contributions
Y.M. provided the original concept for the research. Y.F. and M.Y. designed the study. Y.F., M.F. and S.M. performed the experiments. Y.F., M.F., S.M., Y.K., M.Y. and Y.M. discussed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Fujiwara, Y., Furuta, M., Manabe, S. et al. Imaging mass spectrometry for the precise design of antibody-drug conjugates. Sci Rep 6, 24954 (2016). https://doi.org/10.1038/srep24954
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep24954
- Springer Nature Limited