Abstract
Despite the numerous devoted studies, water at solid interfaces remains puzzling. An ongoing debate concerns the nature of interfacial water at a hydrophilic surface, whether it is more solid-like, ice-like, or liquid-like. To answer this question, a complete picture of the distribution of the water molecule structure and molecular interactions has to be obtained in a non-invasive way and on an ultrafast time scale. We developed a new experimental technique that extends the classical acoustic technique to the molecular level. Using nanoacoustic waves with a femtosecond pulsewidth and an ångström resolution to noninvasively diagnose the hydration structure distribution at ambient solid/water interface, we performed a complete map** of the viscoelastic properties and of the density in the whole interfacial water region at hydrophilic surfaces. Our results suggest that water in the interfacial region possesses mixed properties and that the different pictures obtained up to now can be unified. Moreover, we discuss the effect of the interfacial water structure on the abnormal thermal transport properties of solid/liquid interfaces.
Similar content being viewed by others
Introduction
Solid/liquid-water interface is of fundamental importance in various natural phenomena ranging from surface wetting1, electrolysis2, to protein folding3, wherein the first few water molecular layers govern interface-mediated properties and energy transfer. A variety of techniques, including scanning probe microscopy4,5, surface forces apparatus6, diffraction techniques7,8, nonlinear optical spectroscopy1,9 and infrared and THz spectroscopy10 have been employed to investigate the physical properties of interfacial water. Numerous studies have been devoted to the investigation of the interaction of water with substrate under different conditions, such as the influence of the length of the charge dipoles on a solid surface11,12, the humidity
where η is the dynamic viscosity and ηb is the bulk viscosity. In this region, the high viscosity therefore means that water molecules have a longer relaxation time than bulk water. Indeed, the high density means that the molecules are more closely packed and therefore that their degrees of freedom are restrained. It suggests that the structural relaxation of water molecules takes longer time.
It is important to note that different from numerous reports, we do not observe some clear layering in the density30,31. However a layering effect is suggested from the dam** term profile of Fig. 3(f). Close to the surface, we have a solid like layer with a low viscosity and then the viscosity becomes higher than water similar to a viscous liquid. The observation of this solid-like viscosity behavior closer to the surface is in good agreement with literature32. The absence of layering effect in the density or bulk modulus can be explained by the relatively high roughness of the Al2O3 surface, σ = 5 Å, which is larger than the distance between the expected layers, thus smoothing out the layering information. Even though we calibrate the surface roughness effect by dividing the Al2O3/water interface spectrum by the Al2O3/air interface spectrum, this normalization procedure improve the resolution but is not able to recover the layering effect information. For further discussion on the effect of surface roughness, please refer to supplementary information. One should also note that the density profiles obtained by molecular dynamics simulation usually exhibits an area of few angstrom thicknesses, in which no water molecules can be found33. This area corresponds to the length of the bond between the substrate and water. In our case, we describe water as a succession of thin continuous media. Acoustic waves do not propagate in vacuum but propagate through bonds. A vacuum area without water molecule would mean that acoustic waves cannot propagate and this is against our ultrasonic observation. In ultrasound, the boundary between the solid and water is located in the middle of the bond between water and Al2O3. In order to follow the tradition of molecular dynamics simulation for easier comparison, in Fig. 3(d–f), we have shifted the observed ultrasonic profiles, so that the origin is located at the center of the atomic plane terminating the solid. A more detailed discussion to compare our acoustic results with the conventional view provided by molecular dynamics simulation is given in the supplementary information.
Discussion
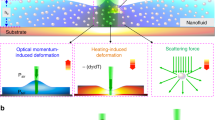
In addition to disclosing the properties of interfacial water, our dynamic study also provides substantial implications for the fundamental nature laws. The observed decay of the acoustic reflectivity at higher frequencies (Inset of Fig. 2(b)) reveals that such phonons are more likely to be transferred into water, compared to the prediction of the continuum elastic theory which neglects the interfacial water structure. It means that when the frequency of phonons becomes high, or in other words when they become sensitive to the interfacial structure, they can more easily transmit to water. In the field of cryogenics, this abnormal heat transport phenomenon has been intensively studied and was found to substantially decrease thermal boundary resistances across a solid/liquid interface, the so-called Kapitza anomaly27,34. Transition from normal-to-anomalous Kapitza resistance typically occurs near 1 K up to 10 K, where the dominant acoustic phonons for heat transport have corresponding frequencies between 90 GHz to 900 GHz, similar to the frequency range of this study as shown in Fig. 1. However, in our study, we only consider longitudinal phonons arriving at the solid/water interface with a normal incidence, which represent only a small part of all phonons participating to the heat conduction (see supplementary information), nevertheless the modified behavior we observed compared to the acoustic mismatch model can also be applied to other phonons. Several mechanisms34 responsible for Kapitza anomaly have been proposed and examined, including liquid boundary layer, conduction electron, phonon attenuation, surface roughness, localized states at the interface and mode conversion. However, conventional phonon spectroscopy cannot perform quantitative studies for specular/diffusive scatterings due to the lack of phase information for the observed acoustic phonons. Recently, we confirmed the dominant role of surface roughness in diffuse phonon scatterings at solid/air and solid/solid interfaces by using nanoultrasonics to measure the frequency-dependent specular scattering probabilities of coherent acoustic phonons at crystal boundaries with well characterized surface conditions20. The surface roughness scattering induces a decrease of the resistance but cannot fully explain the experimental observations. The study reported here clearly indicates that the interfacial liquid structure which acts as an impedance-matching layer is the other dominant mechanism, besides the surface roughness, that gives rise to Kapitza anomaly. Taking advantage of the room-temperature-operated THz-bandwidth coherent phonon source which is capable of distinguishing between specular scatterings and diffuse scatterings20, we thus not only for the first time observed the Kapitza anomaly happening at the solid/water interface, but also further clarify the long-standing puzzles on the origins of Kapitza anomaly. It is also important to know that this Kapitza anomaly phenomenon at the solid/water interface can never be performed at cryogenic temperatures, because at such a low temperature water will not be in the liquid form. This breakthrough should inspire a refreshing way to inspect the problem of energy transports across aqueous solid boundaries, such as the efficient coupling of sub-THz coherent acoustic vibrations from nanoparticles to the surrounding water35. Similar phenomenon has recently been reported by using time-domain thermoreflectance36, showing the thermal conductance of hydrophilic surfaces is 2–3 times higher than that of hydrophobic surfaces. Our result shows that the efficient energy transport across the hydrophilic surface is not merely resulted from the strong bonding but also due to the high mass density and the ordered structure of interfacial water. On the other hand, for hydrophobic surfaces, the high mass density and ice like properties of interfacial water are not expected37. The absence of this feature would make the roughness-scattering the dominant effect to determine the frequency-dependent thermal boundary resistance, while the influence of the interfacial water will be diminished.
Extension from classical to molecular-level ultrasonics is potentially useful for resolving the ongoing debates regarding water due to its unique capability to directly resolve the intermolecular force interactions. The ability of noninvasive probing with picometer perturbations could also enable in situ monitoring of solvation interactions during molecular manipulations for hydrogen energy and biomedical applications. Moreover, this technique will have wide applicability to investigating various solid/liquid interfaces and lead to new insights into the fields of molecular hydrodynamics and condensed matter physics. Indeed, we have demonstrated that interfacial water on a hydrophilic surface can exhibit both liquid- and solid-like features and that the current observations can be unified thanks to the high resolution picture of interfacial water we are giving. Our results also bring a new point of view on the anomalous heat transfer at solid-liquid interface by bringing a microscopic description of the molecular structure at the interface. Those findings may give important insights into thermodynamic studies, such as wetting properties1 and adhesion energy38, on solid/water interfaces.
Methods
Nanoultrasonics
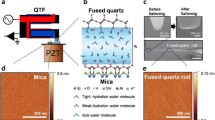
The nanoultrasonic experiment is based on the femtosecond acoustic pulse and echo technique. The mode-locked Ti:Sapphire laser pulse with a 76-MHz repetition rate and a 100-fs pulsewidth was first frequency-doubled by a beta barium borate (BBO) crystal to a wavelength of 400 nm in order to selectively excite the 3 nm In0.1Ga0.9N single quantum well. After second harmonic generation, the laser beam, passed through a blue colour filter and a half-wave plate, was divided into a pump and a probe beams by a polarizing beam splitter. The pump beam was focused on to the InGaN SQW to generate a 400 femtosecond longitudinal acoustic pulse through screening of the piezoelectric field inside the SQW21,22. When the acoustic pulse echo returned and passed through the InGaN SQW again, the strain-pattern-induced optical transient transmissivity change due to the quantum confined Franz-Keldish effect was subsequently measured by the probe beam with an optical delay time controlled by a mechanical translational stage. To enhance the signal to noise ratio, pump beam was chopped by an acousto-optic modulator and the signal of probe beam detected by a photodiode was demodulated by a lock-in amplifier. Typical pump fluence and focused spot diameter were 80 µJ/cm2 and ~30 µm, exciting one-dimensional acoustic pulses with ~10−4 strain in the solid. In order to ensure in situ measurements with and without water loading, the pump and probe beams were incident from the sapphire side of the sample.
Sample preparation
The opto-acoustic transducer is a 3 nm-thick In0.1Ga0.9N single quantum well grown by Metal Organic Chemical Vapor Deposition (MOCVD) on a 2 μm-thick GaN buffer layer with a 300 µm-thick sapphire substrate. On top of the opto-acoustic transducer is an 40 nm-thick p-GaN cap layer with a root-mean-square roughness around 1.2Å on the Ga-terminated GaN(0001) surface. A 10 nm layer of amorphous Al2O3 was deposited by Atomic Layer Deposition (ALD). The water used in this work is liquid chromatography (L. C.) grade. To prevent water evaporation and enable precise positioning, a microfluidic channel was fabricated to facilitate water loading. The microfluidic channel is formed by bonding the sample surface and a BK7 glass by ultraviolet curing of an optical adhesive (NOA81). The BK7 glass surface was laser patterned to define the channel of water flow.
Sample characterization
The surface condition was carefully characterized by AFM, (Veeco innova) before the nanoultrasonic experiments. Topographical images were obtained with a silicon tip in tap** mode. The surface topographical images of the Al2O3 surfaces were then treated by using image processing software to obtain the root-mean-square roughness. 1 × 1, 5 × 5 and 10 × 10 µm2 images on various locations of the sample surfaces were obtained to verify the homogeneity of the roughness. Moreover, the pollution at the sample surfaces was characterized by X-ray photoelectron spectroscopy (VG Scientific ESCALAB 250). The experiments were performed at a pressure of 1.10−10 Torr with a monochromatic X-Ray source at 1486.6 eV. The spot size was 650 µm and the pass energy was 20 eV.