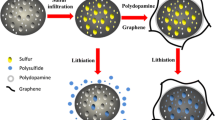
Abstract
We report a simple and efficient approach for fabrication of novel graphene-polysulfide (GPS) anode materials, which consists of conducting graphene network and homogeneously distributed polysulfide in between and chemically bonded with graphene sheets. Such unique architecture not only possesses fast electron transport channels, shortens the Li-ion diffusion length but also provides very efficient Li-ion reservoirs. As a consequence, the GPS materials exhibit an ultrahigh reversible capacity, excellent rate capability and superior long-term cycling performance in terms of 1600, 550, 380 mAh g−1 after 500, 1300, 1900 cycles with a rate of 1, 5 and 10 A g−1 respectively. This novel and simple strategy is believed to work broadly for other carbon-based materials. Additionally, the competitive cost and low environment impact may promise such materials and technique a promising future for the development of high-performance energy storage devices for diverse applications.
Similar content being viewed by others
Introduction
With the increasing global demand for energy in recent years, development of energy conversion and storage technologies faces many opportunities and challenging simultaneously1. Rechargeable lithium-ion batteries (LIBs), one of the most promising candidates for energy storage devices, which have been widely used in portable electronics now request further development to possess super high energy and power density and excellent cycling stability2. Conventional LIBs configuration consists of lithium transition metal oxides or phosphates as the positive electrode (cathode) and graphite-type materials as the negative electrode (anode). Charge storage capability is inherently limited to about 300 mAh g−1, due to the low theoretical specific capacity of the cathode (150–200 mAh g−1) and the anode (372 mAh g−1) materials3,4. Thus, significant research efforts have been focused on searching advanced carbon-based anode materials with enhanced Li ion storage capacity for next-generation LIBs. Up-to-date, various carbon-based materials, such as carbon nanotube5, carbon fiber6, porous carbon7 and their hybrids8 have been well investigated as a possible anode material for LIBs. Graphene and its derivatives have also been considered as a potential electrode material for LIBs19. The C-N bond in the C 1s XPS spectra of G5S and the decreased peak intensity of the oxygen-containing groups in the spectra of the GPS materials further confirm the reduction of GPS materials (Fig. S1)20. Besides C 1s and O 1s peaks for all the samples, S 2p peak at 162.1 eV is clearly seen in the spectra of the GPS materials (Fig. S2), demonstrating the sulfur has been effectively introduced to graphene sheets. To further confirm the bonding configurations of S atoms, the GPS materials were investigated by analyzing the high-resolution S 2p XPS spectra (Fig. 2b and Fig. S3). The S 2p peak of the GPS materials could be fitted as three peaks at binding energies of about 163.4, 164.7 and 168.2 eV, respectively. The two lower energy peaks correspond to the C-S bond21 and S-S bond22, respectively, confirming the presence of chemical bonding between polysulfide and graphene sheet. The weak peak at about 168.2 eV could be attributed to the unwashed sulfate ion in GPS materials23.
The Raman spectra of both GO and GPS materials show two remarkable peaks around 1340 and 1589 cm−1, assigned to the defects or disorders (D band) and the E2g mode (G band) (Fig. S4)Fig. S10). However, the GPS materials exhibit randomly aggregated, thin and crumpled sheets interconnected with each other as shown in Fig. 3b and Fig. S11. This structure may favorably improve the electrochemical performance of the GPS materials, owing to the electrically conducting network of graphene sheets24. Meanwhile, the carbon and sulfur elemental map** clearly demonstrates that sulfur is homogeneously distributed in the GPS materials (Fig. 3c, 3d and Fig. S11).
The morphological analysis of G5S by FESEM and TEM.
(a) FESEM image of G5S. Inset: EDS spectra of GPS showing the presence of sulfur. (b) TEM image of G5S with corresponding elemental map** images of (c) carbon and (d) sulfur in the selected area, indicating the homogeneous distribution of sulfur in G5S.
The lithium storage properties of the GPS materials were further investigated by using the standard GPS/Li half-cell configuration. It can be seen that the cyclic voltammogram (CV) curves of the GPS electrodes are not the same as the carbon-based materials6,26,27, which matches well with the shape of the CV curves. The presence of polysulfide bonds on graphene sheets could still be observed after hundreds of cycles (Fig. S14).
Electrochemical lithium storage performance of G5S electrode.
(a) CV curves of G5S electrode at a scan rate of 0.5 mV s−1 over a voltage range of 0.005 to 3.0 V (vs. Li/Li+). (b) The discharge-charge curves of G5S electrode at a current of 250 mA g−1. (c) Cycling performance of the G5S electrode tested at a current density of 250 mA g−1. (d) Cycling performance of the G5S electrode at various current densities.
The plots of the charge/discharge capacity and coulombic efficiency versus cycle number for the GPS electrodes are evaluated between 0.005 and 3.0 V (vs. Li/Li+) at a current of 250 mA g−1 (Fig. 4c and Fig. S13). It is interesting to observe that the capacity of the GPS electrodes decreases in the first 50 (roughly) cycles and then gradually increases as the cycle number increases, which could be attributed to the activating process of the GPS electrode6,9. Two aspects can be taken into account for this increase in capacity28: (I) the first discharge induces the sudden volume expansion of the active layer due to a large amount of Li-ions insertion into the GPS materials, which may block the further Li-ions transfer from electrode to electrolyte. Therefore, part of the Li2S is possibly left in the GPS materials after the first charge, consequently the capacity decreases in the succeeding cycles. This is also responsible for the low coulombic efficiency in the first cycle. (II) After a number of cycles of Li-ions insertion/extraction, the GPS materials become more expanded and porous as demonstrated by FESEM (Fig. S15), which could effectively promote the electrolyte to access the inner part of the active materials. Thus, the trapped Li2S can re-exposure to the electrolyte and release Li-ions. Meanwhile, the contacting area of the GPS materials with the electrolyte could also be enhanced, resulting in the increase of both the capacity and coulombic efficiency to be over 99%. It is also noted that the capacity is much larger for the longer polysulfide chain, that is capacity of G5S > G4S > G3S > G2S, due to the significantly increased S-S bonds content in the GPS materials (Fig. S9). On the other hand, the enhanced conductivity of graphene with longer polysulfide chain, demonstrated by the electrochemical impedance spectroscopy (EIS) (Fig. S16) could be also responsible for such enhancement of capacity. More importantly, the capacity of G5S can be retained at 1850 mAh g−1 after 380 cycles with coulombic efficiency over 99% per cycle after the first few cycles, which is much higher, actually 5 times of that of the commercially used graphite (372 mAh g−1) and the other reported carbon-based anode materials6,9.
The rate capability is an important parameter for many applications of batteries. Figure 4d shows that the G5S material exhibits high lithium storage and excellent cycling stability even at very high rates. The capacities can sustain as high as 1467, 1620, 1600, 550 and 380 mAh g−1 at charging/discharging rates of 600, 800, 1000, 5000 and 10000 mA g−1 after 360, 440, 500, 1300, 1900 cycles. The ultrahigh capacity, long-term cycling performance and excellent coulombic efficiency of the GPS materials could be attributed to the unique architecture of GPS. First, graphene sheets can be the excellent conductive agent by providing channels for electrons transportation, shortening the diffusion length of Li-ions and fastening Li-ions diffusion from electrolyte to electrode due to its large interfacial area. Second, graphene sheets with the abundant polysulfide bonds can ensure the ultrahigh storage of Li-ions because of the great abilities of storing Li-ions of both two types of reservoirs (graphene and polysulfide bonds). Third, the dissolution of polysulfides can be effectively prevented due to the covalent bond between the polysulfides and graphene. Based on our findings, it can be concluded that high performance carbon-based anode materials for LIBs can be achieved by integrating of polysulfide bonds on the carbon-based materials.
In summary, we have successfully developed a general method for the synthesis of novel GPS anode materials for high performance LIBs, based on the nucleophilic addition reactions between carbonyl and epoxy groups on GO with the polysulfide ions. The unique structural features of the GPS materials such as the homogenous distribution of sulfur on graphene sheets and electrically conducting network of graphene sheets, lead to the superior electrochemical performance in terms of ultrahigh reversible capacity, excellent rate capability and superior long-term cycling performance. We believe that our strategy could be broadly applied to other carbon-based materials, which may open up a new pathway for the realization of various functionalized carbon-based anode materials for high performance LIBs.
Methods
Materials
Graphite powder (< 20 μm), sulfuric acid (H2SO4), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), sodium sulfide nonahydrate (Na2S·9H2O), sulfur (S), SDS, hydrazine monohydrate (NH2NH2·H2O) were purchased from Sigma-Aldrich Pte Ltd and used without further purification.
Preparation of GO suspension
Graphite oxide was synthesized from graphite (Aldrich; < 20 μm) by a modified Hummers' method following the procedures reported earlier29. GO solution (2 mg mL−1) was prepared by ultrasonic exfoliation of graphite oxide (800 mg) into deionized water (400 mL) for 50 min. The pH value of the solution was adjusted to 7 ~ 8 by the addition of a few drops of KOH solution, followed by 10 min centrifugation at 1000 rpm to remove the unexfoliated particles. After that SDS (1 g) was added into the solution and sonicated for another 5 min to obtain GO suspension.
Preparation of sodium polysulfide solutions
Na2S·9H2O (3.85 g) was added into 70 mL deionized water and then S powder was added under continuous stirring and sonication until a transparent solution was achieved. To control the length of the polysulfide chain, different content of S powder was added, such as 0.512 g S for disulfide, 1.024 g S for trisulfide, 1.536 g S for tetrasulfide and 2.048 g S for pentasulfide. The color of the solution changed from bright yellow to yellow-brown with increasing the length of the polysulfide chain through the following reaction:

Preparation of graphene-polysulfide materials
The as-prepared sodium polysulfide solutions were dropwised into the GO suspension under N2 atmosphere and then refluxed at 80°C for 24 h. The product was collected by filtration and washed with deionized water for several times, then redispersed into deionized water (400 mL) by sonication for 5 min. Afterwards, hydrazine monohydrate (7 mL) was added and refluxed at 80°C for 24 h. The obtained graphene-polysulfide, abbreviated as G2S, G3S, G4S and G5S according to the length of the polysulfide chains, respectively, were filtrated and washed with deionized water for several times, then dried in vacuum at 120°C for 12 h.
Characterization
FT-IR was recorded on a NEXUS 670 spectrometer by using pressed KBr pellets. XPS analysis was performed on an ESCALAB MK II X-ray photoelectron spectrometer. FESEM analysis was performed on a JEOL JSM-6700F electron microscope with an EDX spectrometer. TEM measurements were conducted on a JEOL JEM-2010 transmission electron microscope with an accelerating voltage of 200 kV. XRD analysis was performed using a D8 Advanced diffractometer with Cu Kα line (λ = 1.54056 Å). Raman spectra were collected using a WITEC CRM200 Raman system with 532 nm excitation laser. TGA was recorded by a Shimadzu DTG-60H under a heating rate of 10°C/min and a nitrogen flow rate of 50 cm3 min−1.
Electrochemical measurements
The working electrodes were prepared by mixing 80 wt% of the graphene-polysulfide, 10 wt% of acetylene black (Super-P) and 10 wt% of polyvinylidene difluoride dissolved in N-methyl-2-pyrrolidone to form a slurry. The obtained slurry was coated onto a copper foil current collector, which was then dried in vacuum at 90°C for 12 h to remove the solvent. Electrochemical measurements were performed using 2032 coin-type cells with lithium metal as the counter electrode. The electrolyte was 1 mol L−1 LiPF6 in a 50:50 (w/w) mixture of ethylene carbonate and dimethyl carbonate. The coin cells were assembled inside an argon-filled glove box with both moisture and oxygen contents below 0.1 ppm. The galvanostatic charge/discharge tests were performed using a NEWARE battery testing system in the voltage range of 0.005–3.0 V (vs. Li/Li+). The capacity values were calculated based on the weight of GPS materials loaded on the copper foil. CV measurements were performed on CHI 760D electrochemical workstation using a voltage range of 0.005 to 3.0 V (vs. Li/Li+) at a scan rate of 0.5 mV s−1. EIS was conducted on CHI 760D electrochemical workstation.
References
Goodenough, J. B. & Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 22, 587–603 (2009).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 8, 500–506 (2009).
Wang, Z., Zhou, L. & Lou, X. W. Metal Oxide Hollow Nanostructures for Lithium-ion Batteries. Adv. Mater. 24, 1903–1911 (2012).
Zhou, G. et al. A nanosized Fe2O3 decorated single-walled carbon nanotube membrane as a high-performance flexible anode for lithium ion batteries. J. Mater. Chem. 22, 17942–17946 (2012).
Qie, L. et al. Nitrogen-Doped Porous Carbon Nanofiber Webs as Anodes for Lithium Ion Batteries with a Superhigh Capacity and Rate Capability. Adv. Mater. 24, 2047–2050 (2012).
Lee, K. T., Lytle, J. C., Ergang, N. S., Oh, S. M. & Stein, A. Synthesis and Rate Performance of Monolithic Macroporous Carbon Electrodes for Lithium-Ion Secondary Batteries. Adv. Funct. Mater. 15, 547–556 (2005).
Hassoun, J. & Scrosati, B. A High-Performance Polymer Tin Sulfur Lithium Ion Battery. Angew. Chem. Int. Ed. 49, 2371–2374 (2010).
Yan, Y., Yin, Y. X., **n, S., Guo, Y. G. & Wan, L. J. Ionothermal synthesis of sulfur-doped porous carbons hybridized with graphene as superior anode materials for lithium-ion batteries. Chem. Commun. 48, 10663–10665 (2012).
Ji, H. et al. Ultrathin Graphite Foam: A Three-Dimensional Conductive Network for Battery Electrodes. Nano Lett. 12, 2446–2451 (2012).
Yang, S. et al. Porous Iron Oxide Ribbons Grown on Graphene for High Performance Lithium Storage. Sci. Rep. 2, 427 (2012).
Liang, Y., Tao, Z. & Chen, J. Organic Electrode Materials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2, 742–769 (2012).
Fanous, J., Wegner, M., Grimminger, J., Andresen, A. & Buchmeiser, M. R. Structure-Related Electrochemistry of Sulfur-Poly(acrylonitrile) Composite Cathode Materials for Rechargeable Lithium Batteries. Chem. Mater. 23, 5024–5028 (2011).
Krishnan, P., Advani, S. G. & Prasad, A. K. Synthesis and evaluation of polythiocyanogen (SCN)x as a rechargeable lithium-ion battery electrode material. J. Power Sources 196, 7755–7759 (2011).
Wang, H. et al. Graphene-Wrapped Sulfur Particles as a Rechargeable Lithium-Sulfur Battery Cathode Material with High Capacity and Cycling Stability. Nano Lett. 11, 2644–2647 (2011).
Marcano, D. C. et al. Improved Synthesis of Graphene Oxide. ACS Nano. 4, 4806–4814 (2010).
Brownridge, S. et al. The Highest Bond Order Between Heavier Main-Group Elements in an Isolated Compound? Energetics and Vibrational Spectroscopy of S2I4(MF6)2 (M = As, Sb). Inorg. Chem. 44, 1660–1671 (2005).
Zhang, S. C., Zhang, L., Wang, W. K. & Xue, W. J. A Novel cathode material based on polyaniline used for lithium/sulfur secondary battery. Synth. Met. 160, 2041–2044 (2010).
Park, S. et al. Chemical structures of hydrazine-treated graphene oxide and generation of aromatic nitrogen do**. Nat. Commun. 3, 638 (2012).
Stankovich, S. et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007).
Boyer, C. et al. The stabilization and bio-functionalization of iron oxide nanoparticles using heterotelechelic polymers. J. Mater. Chem. 19, 111–123 (2009).
Kohli, P., Taylor, K. K., Harris, J. J. & Blanchard, G. J. Assembly of Covalently-Coupled Disulfide Multilayers on Gold. J. Am. Chem. Soc. 120, 11962–11968 (1998).
Shao, X., Tian, J., Xue, Q. & Ma, C. Fabrication of MoO3 nanoparticles on an MoS2 template with (C4H9Li)MoS2 exfoliation. J. Mater. Chem. 13, 631–633 (2003).
Ai, W. et al. Benzoxazole and benzimidazole heterocycle-grafted graphene for high-performance supercapacitor electrodes. J. Mater. Chem. 22, 23439–23446 (2012).
Zhang, J. et al. Poly(ethene-1,1,2,2-tetrathiol): Novel cathode material with high specific capacity for rechargeable lithium batteries. J. Power Sources 186, 496–499 (2009).
Poizot, P., Laruelle, S., Grugeon, S., Dupont, L. & Tarascon, J. M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 407, 496–499 (2000).
Poizot, P., Laruelle, S., Grugeon, S. & Tarascon, J. M. Rationalization of the low-potential reactivity of 3d-metal-based inorganic compounds toward Li. J. Electrochem. Soc. 149, A1212–A1217 (2002).
Ji, H. X. et al. Self-Wound Composite Nanomembranes as Electrode Materials for Lithium Ion Batteries. Adv. Mater. 22, 4591–4595 (2010).
Shang, J. et al. The Origin of Fluorescence from Graphene Oxide. Sci. Rep. 2, 792 (2012).
Acknowledgements
This work is supported by the Singapore National Research Foundation under NRF RF Award No. NRFRF2010-07. LHX and WH thank the support of the “973” project (No. 2009CB930600, 2012CB933301), NNSFC (Grants No. 21144004, 61136003, 51173081, 20974046, 50428303), the Program for New Century Excellent Talents in University (NCET-11-0992), the Ministry of Education of China (No. IRT1148), the NSF of Jiangsu Province (Grants No. SBK201122680, 11KJB510017, BK2008053, 11KJB510017, BZ2010043 and No.BK2009025) and NUPT (No. NY210030 and NY211022). WA thanks the help of Dr. Shi Chen and Mr. Ng Chin Fan for the XPS measurement of the G5S after 400 cycles.
Author information
Authors and Affiliations
Contributions
W.A. and T.Y. initialled the project, conceived and designed the experiments; W.A. and T.Y. performed the battery tests; W.A., Z.Z.D. and L.H.X. conducted FTIR and XPS measurements. W.A., Z.Y.Z., J.Q.L. and H.Z. did the TEM. W.A. and T.Y. collected and analysed the data and co-wrote the paper with W.H. and Y.H.H. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ai, W., **e, L., Du, Z. et al. A Novel Graphene-Polysulfide Anode Material for High-Performance Lithium-Ion Batteries. Sci Rep 3, 2341 (2013). https://doi.org/10.1038/srep02341
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02341
- Springer Nature Limited
This article is cited by
-
Chromium stabilization by polysulfide supported nZVI@biochar in contaminated soil: Cr bioavailability and stabilization mechanism
Journal of Central South University (2024)
-
Application and research of current collector for lithium-sulfur battery
Ionics (2022)
-
Confined SnO2 quantum-dot clusters in graphene sheets as high-performance anodes for lithium-ion batteries
Scientific Reports (2016)
-
A novel quinone-based polymer electrode for high performance lithium-ion batteries
Science China Materials (2016)
-
Single Source Precursor-based Solvothermal Synthesis of Heteroatom-doped Graphene and Its Energy Storage and Conversion Applications
Scientific Reports (2014)