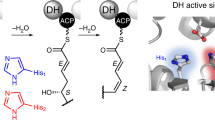
Abstract
Modular polyketide synthases (type I PKSs) are biosynthetic assembly lines for synthesizing a diverse array of natural products. While most PKSs exhibit a linear module architecture, the PKS system for lankacidin-type natural products contains only five modules but carries out eight rounds of polyketide extension, challenging the collinearity rule. Here we show the distinct domain architecture of the polyketide synthase enzyme, CheC, which is central to chejuenolide biosynthesis. CheC not only dissociates from and interacts with both the preceding and succeeding PKS enzymes, creating two linear modules, but also independently assembles an unconventional module, facilitating multiple rounds of polyketide extension in the biosynthetic process. We also unveiled missing functions of certain redundant and absent domains within PKSs, fully elucidating the polyketide assembly process for lankacidin-like natural products. These findings not only reveal the biosynthetic pathway for lankacidin- and chejuenolin-type natural products but also enrich the diverse functions of PKSs, setting the stage for future rational design of PKSs.







Similar content being viewed by others
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. All the DNA or protein sequences were deposited in GenBank, and the accession numbers were listed in figure legends.
References
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009).
Nivina, A., Yuet, K. P., Hsu, J. & Khosla, C. Evolution and diversity of assembly-line polyketide synthases. Chem. Rev. 119, 12524–12547 (2019).
Rawlings, B. J. Type I polyketide biosynthesis in bacteria (Part A–erythromycin biosynthesis). Nat. Prod. Rep. 18, 190–227 (2001).
Benjamin, D., Colombi, M., Moroni, C. & Hall, M. N. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 10, 868–880 (2011).
Ikeda, H. & Ōmura, S. Avermectin biosynthesis. Chem. Rev. 97, 2591–2610 (1997).
Nicolaou, K. C., Roschangar, F. & Vourloumis, D. Angew. Chem. Int. Ed. 37, 2014–2045 (1998).
Liou, G. F. & Khosla, C. Building-block selectivity of polyketide synthases. Curr. Opin. Chem. Biol. 7, 279–284 (2003).
Dutta, S. et al. Structure of a modular polyketide synthase. Nature 510, 512–517 (2014).
Fischbach, M. A. & Walsh, C. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev. 106, 3468–3496 (2006).
Whicher, J. R. et al. Structural rearrangements of a polyketide synthase module during its catalytic cycle. Nature 510, 560–564 (2014).
Keatinge-Clay, A. T. The structures of type I polyketide synthases. Nat. Prod. Rep. 29, 1050–1073 (2012).
Smith, S. & Tsai, S.-C. The type I fatty acid and polyketide synthases: a tale of two megasynthases. Nat. Prod. Rep. 24, 1041–1072 (2007).
Eng, C. H. et al. ClusterCAD: a computational platform for type I modular polyketide synthase design. Nucleic Acids Res. 46, D509–D515 (2018).
Piel, J. A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc. Natl Acad. Sci. USA 99, 14002–14007 (2002).
Cheng, Y. Q., Tang, G. L. & Shen, B. Type I polyketide synthase requiring a discrete acyltransferase for polyketide biosynthesis. Proc. Natl Acad. Sci. USA 100, 3149–3154 (2003).
Piel, J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 27, 996–1047 (2010).
Helfrich, E. J. N. & Piel, J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 33, 231–316 (2016).
Kosol, S., Jenner, M., Lewandowski, J. R. & Challis, G. L. Protein–protein interactions in trans-AT polyketide synthases. Nat. Prod. Rep. 35, 1097–1109 (2018).
Nguyen, T. A. et al. Exploiting the mosaic structure of trans-acyltransferase polyketide synthases for natural product discovery and pathway dissection. Nat. Biotechnol. 26, 225–233 (2008).
Helfrich, E. J. N. et al. Automated structure prediction of trans-acyltransferase polyketide synthase products. Nat. Chem. Biol. 15, 813–821 (2019).
Austin, M. B. et al. Biosynthesis of dictyostelium discoideum differentiation-inducing factor by a hybrid type I fatty acid-type III polyketide synthase. Nat. Chem. Biol. 2, 494–502 (2006).
Shen, B. Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr. Opin. Chem. Biol. 7, 285–295 (2003).
Chen, H. & Du, L. Iterative polyketide biosynthesis by modular polyketide synthases in bacteria. Appl. Microbiol. Biotechnol. 100, 541–557 (2016).
Wang, J., Deng, Z., Liang, J. & Wang, Z. Structural enzymology of iterative type I polyketide synthases: various routes to catalytic programming. Nat. Prod. Rep. 40, 1498–1520 (2023).
Gaumann, E. et al. Stoffwechselprodukte von Actinomyceten. 21. Mitteilung. Lankamycin und Lankacidin. Helv. Chim. Acta 43, 601–606 (1960).
Uramoto, M. et al. The structures of bundlin A (lankacidin) and bundlin B. Tetrahedron Lett. 10, 2249–2254 (1969).
Choi, Y.-H. et al. Chejuenolides A and B, new macrocyclic tetraenes from the marine bacterium Hahella chejuensis. Tetrahedron Lett. 50, 7128–7131 (2008).
Suwa, M. et al. Identification of two polyketide synthase gene clusters on the linear plasmid pSLA2-L in Streptomyces rochei. Gene 246, 123–131 (2000).
Ng, B. G., Han, J. W., Lee, D. W., Choi, G. J. & Kim, B. S. The chejuenolide biosynthetic gene cluster harboring an iterative trans-AT PKS system in Hahella chejuensis strain MB-1084. J. Antibiot. 71, 495–505 (2018).
Arakawa, K., Sugino, F., Kodama, K., Ishii, T. & Kinashi, H. Cyclization mechanism for the synthesis of macrocyclic antibiotic lankacidin in Streptomyces rochei. Chem. Biol. 12, 249–256 (2005).
Tatsuno, S., Arakawa, K. & Kinashi, H. Analysis of modular-iterative mixed biosynthesis of lankacidin by heterologous expression and gene fusion. J. Antibiot. 60, 700–708 (2007).
Dorival, J. et al. Insights into a dual function amide oxidase/macrocyclase from lankacidin biosynthesis. Nat. Commun. 9, 3998 (2018).
Dickschat, J. S. et al. An additional dehydratase-like activity is required for lankacidin antibiotic biosynthesis. ChemBioChem 12, 2408–2412 (2011).
Quadri, L. E. N. et al. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 37, 1585–1595 (1998).
Zheng, K., Shen, D. & Hong, R. Biomimetic synthesis of lankacidin antibiotics. J. Am. Chem. Soc. 139, 12939–12942 (2017).
Zhang, B., Zheng, K. & Hong, R. Biomimetic synthesis of chejuenolides A-C by a cryptic lactone-based macrocyclization: stereochemical implications in biosynthesis. ACS Cent. Sci. 9, 84–92 (2023).
Jiang, H. et al. The role of tandem acyl carrier protein domains in polyunsaturated fatty acid biosynthesis. J. Am. Chem. Soc. 130, 6336–6337 (2008).
Wang, Z. L. et al. De novo design and implementation of a tandem acyl carrier protein domain in a type I modular polyketide synthase. ACS Chem. Biol. 13, 3072–3077 (2018).
Zhu, Z. W. et al. Expanding the product portfolio of fungal type I fatty acid synthases. Nat. Chem. Biol. 13, 360–362 (2017).
Bunnak, W. et al. SAXS reveals highly flexible interdomain linkers of tandem acyl carrier protein–thioesterase domains from a fungal nonreducing polyketide synthase. FEBS Lett. 595, 133–144 (2020).
Zhang, B. et al. A long-range acting dehydratase domain as the missing link for C17-dehydration in iso-migrastatin biosynthesis. Angew. Chem. Int. Ed. 56, 7247–7251 (2017).
Zhai, G. et al. Insights into azalomycin F assembly-line contribute to evolution-guided polyketide synthase engineering and identification of intermodular recognition. Nat. Commun. 14, 612 (2023).
Luo, M. et al. The mechanism of dehydrating bimodules in trans-acyltransferase polyketide biosynthesis: a showcase study on hepatoprotective hangtaimycin. Angew. Chem. Int. Ed. 60, 19139–19143 (2021).
He, J. & Hertweck, C. Functional analysis of the aureothin iterative type I polyketide synthase. ChemBioChem 6, 908–912 (2005).
Pfeifer, B. A. et al. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291, 1790–1792 (2001).
Horsman, G. P., Van Lanen, S. G. & Shen, B. Iterative type I polyketide synthases for enediyne core biosynthesis. Methods Enzymol. 459, 97–112 (2009).
Ma, S. M. et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 326, 589–592 (2009).
Moriguchi, T., Ebizuka, Y. & Fujii, I. Analysis of subunit interactions in the iterative type I polyketide synthase ATX from Aspergillus terreus. ChemBioChem 7, 1869–1874 (2006).
Cochrane, R. V. K. et al. Comparison of 10,11-dehydrocurvularin polyketide synthases from Alternaria cinerariae and Aspergillus terreus highlights key structural motifs. ChemBioChem. 16, 2479–2483 (2015).
Cox, R. J. Curiouser and curiouser: progress in understanding the programming of iterative highly-reducing polyketide synthases. Nat. Prod. Rep. 40, 9–27 (2023).
Mabesoone, M. F. J. et al. Evolution-guided engineering of trans-acyltransferase polyketide synthases. Science 383, 1312–1317 (2024).
Acknowledgements
This research was financially supported by National Key R&D Program of China (2018YFA0902000 to R.H.J., 2022YFC2303100 to H.M.G. and 2022YFC2804100 to H.M.G.) and National Natural Science Foundation of China (81925033 to H.M.G., 22193071 to H.M.G., 81803380 to B.Z., 22207052 to Z.P.M., 81991522 to B.Z. and 22107048 to J.S.).
Author information
Authors and Affiliations
Contributions
Z.P.M., B.Z., Z.X.P. and S.Y.M. carried out experiments; Z.F.X., J.S. and R.H.J. assisted in NMR and MS data measurement and analysis; Z.-J.Y. and B.-B.H. assisted in chemical synthesis; R.X.T. contributed materials and equipment; Z.P.M., B.Z. and H.M.G. wrote the paper. B.Z. and H.M.G. supervised the work. All authors discussed the results and analysed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Yuhui Sun, Kira Weissman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Biosynthetic logic of cis-AT PKS and trans-AT PKS.
Comparison of canonical domain architectures in cis-AT PKS (a) and trans-AT PKS (b) for assembly of a fictional polyketide (dashed box). KS, ketosynthase domain; AT(Mal.), acyltransferase recognized malonyl-CoA; AT(mMal.), acyltransferase recognized methylmalonyl-CoA; ACP, acyl carrier protein domain; KR, ketoreductase domain; DH, dehydratase domain; ER, enoyl reductase; MeT, methyltransferase domain; TE, thioesterase domain; GNAT, GCN5-related AT.
Extended Data Fig. 2 LC-MS analysis of the one-pot enzymatic reconstitution of chejuenolin (IV).
In vitro enzymatic reaction of CheABCDEFG with glycine as start unit in the presence of AcCoA, ATP, MgCl2, malonyl-CoA, NADPH, SAM and FAD. The EIC analyzed used m/z 432.2381 with ± 0.005 mass tolerance. Each experiment was repeated three times, and similar results were obtained.
Extended Data Fig. 3 LC-MS analysis of the one-pot enzymatic reconstitution of 10.
The EIC analyzed used m/z 432.2392 with ± 0.005 mass tolerance. Each experiment was repeated three times, and similar results were obtained.
Extended Data Fig. 4 LC-MS detection of elongated polyketide chains through in vitro enzymatic reaction using 6b as substrate.
LC-MS analysis of the elongated polyketide chains released from CheF after KOH hydrolysis. The EIC analyzed used exact calculated masses with ± 0.005 tolerance. Each experiment was repeated three times, and similar results were obtained.
Extended Data Fig. 5 LC-MS analysis of the function of tandem ACP domains in CheC.
i) As a positive control, N-acetylglycine as a start unit reacts with CheA-D and CheF-G in the presence of malonyl-CoA, NADPH and SAM. ii) CheC was replaced by truncated CheC-KR-MeT, CheC-ACP1-ACP2 and CheC-KS-DH at the same reaction with i). iii-iv) The truncated CheC-ACP1-ACP2 was replaced by site-directed mutant CheC-ACP1-S871A and site-directed mutant CheC-ACP2-S974A at the same reaction with iii), respectively. v) As a negative control, N-acetylglycine works with the CheC(ACP1-S871A-ACP2-S974A) instead of CheC at the same reaction with i). vi-vii) The truncated CheC-ACP1-ACP2 was replaced by truncated CheC-ACP1 and truncated CheC-ACP2 at the same reaction with ii, respectively. viii) standard of 10. The EIC analyzed used m/z 432.2392 with ± 0.005 mass tolerance. Each experiment was repeated three times, and similar results were obtained.
Extended Data Fig. 6 LC-MS analysis of the putative trans-acting methylation.
i) Thiophenol derivative 7b reacts with CheDFG and CheC-MeT in the presence of cofactors, malonyl-CoA, NADPH and SAM, followed by KOH hydrolysis; ii) Based on reaction i, the expressed CheC-MeT domain and SAM was removed. The EIC analyzed used exact calculated masses with ± 0.005 tolerance. Each experiment was repeated three times, and similar results were obtained.
Extended Data Fig. 7 Fragmentation spectrum of the proposed polyketide 8a and 8c produced by enzymatic reaction.
a/b, MS/MS2 fragmentation spectrum of 8a and 8c, the key product ions were highlighted and the clear signatures of Δ14 Da were observed. Each experiment was repeated three times, and similar results were obtained.
Extended Data Fig. 8 LC-MS analysis of the function of the redundant KS-ACP domains in the final module.
a, LC-MS analysis of the function of CheF-KS2 and CheG-KS domains. i) Thiophenol derivative 7b reacts with CheD, CheF-G and CheC-MeT in the presence of Mal-CoA, SAM and NADPH to product 10. ii) CheF was replaced by CheF (KS2-C1952A) in the same reaction as i). iii) CheG was replaced by CheG (KS-C258A) in the same reaction as i). iv) CheF and CheG were both replaced by CheF (KS2-C1952A) and CheG (KS-C258A) int the same reaction as i). b, LC-MS analysis of the function of CheG-ACP1 and CheG-ACP2 domains. i) Thiophenol derivative 7b reacts with CheD, CheF-G and CheC-MeT in the presence of Mal-CoA, SAM and NADPH to product 10. ii) CheG was replaced by CheG (ACP1-S35A) in the same reaction as i). iii) CheG was replaced by CheG (ACP2-S715A) in the same reaction as i). iv) CheG weas replaced by CheG (ACP1-S35A-ACP2-S715A) into the same reaction as i). The EIC analyzed used m/z 432.2392 with ± 0.005 mass tolerance. Each experiment was repeated three times, and similar results were obtained.
Supplementary information
Supplementary Information
Supplementary experimental procedures, Figs. 1–103 and Tables 1–6.
Supplementary Data 1
Statistical source data.
Supplementary Data 2
Statistical source data.
Supplementary Data 3
Statistical source data.
Supplementary Data 4
Statistical source data.
Supplementary Data 5
Statistical source data.
Supplementary Data 6
Statistical source data.
Supplementary Data 7
Statistical source data.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mai, Z.P., Zhang, B., Pang, Z.X. et al. Insight into the role of a trans-AT polyketide synthase in the biosynthesis of lankacidin-type natural products. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00599-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00599-1
- Springer Nature Limited