Abstract
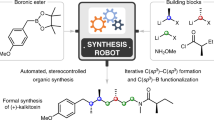
Automated iterative small-molecule synthesis has the potential to advance and democratize the discovery of new medicines, materials and many other classes of functional chemical matter. To date, however, this approach has been limited because each carbon–carbon bond-forming step takes about a day. Here we report a next-generation small-molecule synthesizer that operates an order of magnitude faster than previous systems through improvements in both chemistry and engineering. Key advances include the discovery that rapid Suzuki–Miyaura cross-couplings under homogeneous conditions, although not tolerated by N-methyliminodiacetic acid boronates, are fully compatible with their more stable tetramethyl-N-methyliminodiacetic acid boronate counterparts, and the development of optimized cartridges for rapid catch-and-release purification. These findings move the field of small-molecule synthesis a step closer to democratizing its core discovery engine.





Similar content being viewed by others
Data availability
The data and methods that support the findings of this study are available in the Supplementary Information, which describes the manual and automated synthesis methods, kinetic studies and control experiments.
Change history
11 June 2024
A Correction to this paper has been published: https://doi.org/10.1038/s44160-024-00601-w
References
Merrifield, R. B. Automated synthesis of peptides. Science 150, 178–185 (1965).
Caruthers, M. H. Gene synthesis machines: DNA chemistry and its uses. Science 230, 281–285 (1985).
Mitchell, A. R. Bruce Merrifield and solid-phase peptide synthesis: a historical assessment. Pept. Sci. 90, 175–184 (2008).
Tian, J., Li, Y., Ma, B., Tan, Z. & Shang, S. Automated peptide synthesizers and glycoprotein synthesis. Front. Chem. 10, 896098 (2022).
Wang, L. et al. Therapeutic peptides: current applications and future directions. Signal Transduct. Target. Ther. 7, 48–74 (2022).
Trevino, V., Falciani, F. & Barrera-Saldaña, H. A. DNA microarrays: a powerful genomic tool for biomedical and clinical research. Mol. Med. 13, 527–541 (2007).
Gibson, D. G. et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56 (2010).
Khorana, H. G. Total synthesis of a gene. Science 203, 614–625 (1979).
Kent, S. B. H. Total chemical synthesis of proteins. Chem. Soc. Rev. 38, 338–351 (2009).
Gigoux, M. et al. Calreticulin mutant myeloproliferative neoplasms induce MHC-I skewing, which can be overcome by an optimized peptide cancer vaccine. Sci. Transl. Med. 14, eaba4380 (2022).
Kwon, P. S. et al. Designer DNA architecture offers precise and multivalent spatial pattern-recognition for viral sensing and inhibition. Nat. Chem. 12, 26–35 (2020).
Plante, O. J., Palmacci, E. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001).
Coste, J., Le-Nguyen, D. & Castro, B. PyBOP®: a new peptide coupling reagent devoid of toxic by-product. Tetrahedron Lett. 31, 205–208 (1990).
Saebi, A. et al. Rapid single-shot synthesis of the 214 amino acid-long N-terminal domain of pyocin S2. ACS Chem. Biol. 18, 518–527 (2023).
Pedersen, S. L., Tofteng, A. P., Malik, L. & Jensen, K. J. Microwave heating in solid-phase peptide synthesis. Chem. Soc. Rev. 41, 1826–1844 (2012).
Danglad-Flores, J. et al. Microwave-assisted automated glycan assembly. J. Am. Chem. Soc. 143, 8893–8901 (2021).
Beck, H., Härter, M., Haß, B., Schmeck, C. & Baerfacker, L. Small molecules and their impact in drug discovery: a perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory. Drug Discov. Today 27, 1560–1574 (2022).
Trobe, M. & Burke, M. D. The molecular industrial revolution: automated synthesis of small molecules. Angew. Chem. Int. Ed. 57, 4192–4214 (2018).
Gillis, E. P. & Burke, M. D. A simple and modular strategy for small-molecule synthesis: iterative Suzuki–Miyaura coupling of B-protected haloboronic acid building blocks. J. Am. Chem. Soc. 129, 6716–6717 (2007).
Li, J., Grillo, A. S. & Burke, M. D. From synthesis to function via iterative assembly of N-methyliminodiacetic acid boronate building blocks. Acc. Chem. Res. 48, 2297–2307 (2015).
Lehmann, J. W., Blair, D. J. & Burke, M. D. Towards the generalized iterative synthesis of small molecules. Nat. Rev. Chem. 2, 0115 (2018).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Li, S. et al. Using automated synthesis to understand the role of side chains on molecular charge transport. Nat. Commun. 13, 2102 (2022).
Wu, T. C. et al. A materials acceleration platform for organic laser discovery. Adv. Mater. 35, 2207070 (2023).
Angello, N. H. et al. Closed-loop optimization of general reaction conditions for heteroaryl Suzuki–Miyaura coupling. Science 378, 399–405 (2022).
Blair, D. J. et al. Automated iterative Csp3–C bond formation. Nature 604, 92–97 (2022).
Delaney, C. P., Kassel, V. M. & Denmark, S. E. Potassium trimethylsilanolate enables rapid, homogeneous Suzuki–Miyaura cross-coupling of boronic esters. ACS Catal. 10, 73–80 (2020).
Delaney, C. P. et al. Potassium trimethylsilanolate-promoted, anhydrous Suzuki–Miyaura cross-coupling reaction proceeds via the “boronate mechanism”: evidence for the alternative fork in the trail. J. Am. Chem. Soc. 144, 4345–4364 (2022).
Kassel, V. M., Hanneman, C. M., Delaney, C. P. & Denmark, S. E. Heteroaryl–heteroaryl, Suzuki–Miyaura, anhydrous cross-coupling reactions enabled by trimethyl borate. J. Am. Chem. Soc. 143, 13845–13853 (2021).
Roush, W. R. & Brown, B. B. Application of the steric directing group strategy to the stereoselective synthesis of the octahydronaphthalene substructure of kijanolide and tetronolide. J. Am. Chem. Soc. 115, 2268–2278 (1993).
Cox, P. A. et al. Base-catalyzed Aryl-B(OH)2 protodeboronation revisited: from concerted proton transfer to liberation of a transient aryl anion. J. Am. Chem. Soc. 139, 13156–13165 (2017).
Fischer, F. C. & Havinga, E. Thermal and photoinduced deboronations of some pyridine- and benzeneboronate anions. Recl. Trav. Chim. Pays-Bas 93, 21–24 (1974).
Dreos, R. et al. A molecular box derived from cobaloxime units held together by 4-pyridinylboronic acid residues. Inorg. Chem. 40, 5536–5540 (2001).
Hayes, H. L. D. et al. Protodeboronation of (hetero)arylboronic esters: direct versus prehydrolytic pathways and self-/auto-catalysis. J. Am. Chem. Soc. 143, 14814–14826 (2021).
Li, S. et al. Transition between nonresonant and resonant charge transport in molecular junctions. Nano Lett. 21, 8340–8347 (2021).
Woerly, E. M., Roy, J. & Burke, M. D. Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction. Nat. Chem. 6, 484–491 (2014).
Lee, C. F. et al. Amine hemilability enables boron to mechanistically resemble either hydride or proton. Nat. Chem. 10, 1062–1070 (2018).
Fasano, V. et al. Automated stereocontrolled assembly-line synthesis of organic molecules. Nat. Synth. 1, 902–907 (2022).
Wang, G., Ang, H. T., Dubbaka, S. R., O’Neill, P. & Wu, J. Multistep automated synthesis of pharmaceuticals. Trends Chem. 5, 432–445 (2023).
Acknowledgements
This work was supported by the Molecule Maker Lab Institute, an AI Research Institutes programme supported by the US National Science Foundation under grant number 2019897 (M.D.B.). N.H.A. was supported by the Department of Defense (DoD) through the National Defense Science and Engineering Graduate (NDSEG) Fellowship Program. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect those of the NSF. The authors gratefully acknowledge S. Denmark, V. Kassel and M. Bock for advice regarding TMSOK-promoted couplings. D.J.B. thanks the American Lebanese Syrian Associated Charities (ALSAC) and St Jude Children’s Research Hospital for support.
Author information
Authors and Affiliations
Contributions
M.D.B., W.W., N.H.A. and D.J.B. conceived this project. W.W., N.H.A., D.J.B., T.T.-E., W.H.K., K.N.S.M., A.J.L., J.M.B. and M.D.B. designed and executed the experiments. W.W., N.H.A., D.J.B. and M.D.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The University of Illinois has filed patent applications related to MIDA and TIDA boronates with M.D.B., W.W., N.H.A., D.J.B. and T.T.-E. as inventors. The other authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Timothy Cernak and Jie Wu for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary Sections 1–4, Figs. 1–34 and Tables 1–7.
Supplementary Data 1
Source data for graphs in the Supplementary Figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Angello, N.H., Blair, D.J. et al. Rapid automated iterative small-molecule synthesis. Nat. Synth (2024). https://doi.org/10.1038/s44160-024-00558-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-024-00558-w
- Springer Nature Limited