Abstract
The aim of this study was to investigate the relationship between source-specific ambient particulate air pollution concentrations and the incidence of dementia. The study encompassed 70,057 participants from the Västerbotten intervention program cohort in Northern Sweden with a median age of 40 years at baseline. High-resolution dispersion models were employed to estimate source-specific particulate matter (PM) concentrations, such as PM10 and PM2.5 from traffic, exhaust, and biomass (mainly wood) burning, at the residential addresses of each participant. Cox regression models, adjusted for potential confounding factors, were used for the assessment. Over 884,847 person-years of follow-up, 409 incident dementia cases, identified through national registers, were observed. The study population’s average exposure to annual mean total PM10 and PM2.5 lag 1–5 years was 9.50 µg/m3 and 5.61 µg/m3, respectively. Increased risks were identified for PM10-Traffic (35% [95% CI 0–82%]) and PM2.5-Exhaust (33% [95% CI − 2 to 79%]) in the second exposure tertile for lag 1–5 years, although no such risks were observed in the third tertile. Interestingly, a negative association was observed between PM2.5-Wood burning and the risk of dementia. In summary, this register-based study did not conclusively establish a strong association between air pollution exposure and the incidence of dementia. While some evidence indicated elevated risks for PM10-Traffic and PM2.5-Exhaust, and conversely, a negative association for PM2.5-Wood burning, no clear exposure–response relationships were evident.
Similar content being viewed by others
Introduction
The rapid expansion of aging populations presents significant public health challenges, particularly the rising incidence of neurological diseases closely associated with age1. Neurological disorders rank among the leading causes of morbidity and mortality globally, constituting 10% of the overall disease burden. Dementia, a broad term encompassing a range of neurological disorders characterized by the progressive decline in memory, language, cognitive abilities, behavior, and problem-solving skills, ultimately impeding daily functioning and social interactions, contributed to approximately 10% of neurological disability-adjusted life years (DALYs) worldwide in 20162. Alzheimer’s disease (AD) and non-Alzheimer dementia (NAD), such as vascular dementia (VaD), stand as some of the most prevalent forms of dementia3. Currently, an estimated 160,000 individuals in Sweden live with dementia, equivalent to 1.5% of the entire population. This not only imposes a significant physical and emotional burden on patients and their families but also places substantial strain on the healthcare system and society as a whole4.
Given its multifaceted origins, dementia is influenced by a range of genetic, behavioral, and environmental risk factors. With no effective pharmaceutical treatments available, the identification and targeting of modifiable risk factors, specifically those related to behavior and the environment, assume paramount importance. Among the potential environmental factors, air pollution has garnered increasing attention in epidemiological research. In fact, The Lancet Commission on Dementia Prevention, Intervention, and Care has identified air pollution as a significant risk factor for dementia5. According to this report, approximately 2% of all dementia cases can be attributed to air pollution. Ambient air pollution constitutes a persistent, long-term exposure that often spans extensive regions, particularly urban areas, affecting a large segment of the population throughout their lifetimes. Consequently, it stands as one of the few environmental risk factors that can be modified at the population level through air quality control measures and mitigation interventions.
Despite a growing body of evidence linking air pollution exposure to dementia, the precise underlying biological mechanisms remain incompletely understood. Proposed pathways include the direct transport of pollutants to the brain through the olfactory bulb, as well as the induction of systemic inflammation and oxidative stress6. A pioneering study analyzing MRI data in both children and dogs revealed damage to the prefrontal cortical regions of the brain in individuals exposed to high levels of air pollution7. Experimental investigations have also suggested that neuroinflammation and the deposition of ultrafine particles in the brain can lead to enlarged Virchow–Robin spaces, gliosis, and frontal lesions accompanied by vascular pathology in exposed individuals7,8,9. Moreover, animal model studies have provided compelling evidence of the harm associated with air pollution, including impaired blood–brain barrier function, white matter lesions, neuronal degeneration, oxidative damage, glial activation, and neuroinflammation10,11. Even short-term exposure to air pollution has been shown to elicit inflammatory responses in brain tissue12 and disrupt functional connectivity in the default mode network13. In recent years, a substantial body of evidence has thus emerged regarding the role of air pollution in neurodegenerative pathology14,15. Notably, an expanding corpus of epidemiological research has explored the impact of fine particulate matter with an aerodynamic diameter of ≤ 2.5 µm (PM2.5 on cognitive function and dementia in the elderly population16,17,18. Several studies19,20,21,22 have highlighted how, for example, PM2. 5 is able to cross the blood-alveolar and blood–brain barriers. Because PM2.5 is able to promote cellular oxidative stress and systemic inflammatory responses involved in the processes that lead to the onset of respiratory diseases, cardiovascular and neurodegenerative diseases, it represents an important environmental risk factor for public health. It has also been suggested that airborne particulate matter (PM) exposure accelerates brain aging, particularly in ɛ4 carriers, possibly through increased cerebral Aβ production and alterations in hippocampal CA1 neurons and glutamate receptor subunits23. In a study with 3029 participants, a 3 µg/m3 increase in particles with aerodynamic diameters less than 10 µm (PM10) over 10 years was, furthermore, associated with a 1.80% higher baseline level of Aβ1-40 (95% confidence interval (CI) 0.22%, 3.40%), whereas in repeated measures analyses, the association was stronger with a 5.20% increase (95% CI 3.69%, 6.72%)24. Similar associations were found for fine particles with an aerodynamic diameter of ≤ 2.5 µm (PM2.5) (interquartile range (IQR) 2 µg/m3) and nitrogen dioxide (NO2) (IQR 7 ppb) in repeated measures analyses24.
To establish a causal relationship between long-term air pollution exposure and dementia, well-designed longitudinal observational studies are essential. A comprehensive review focusing on the epidemiological evidence concerning the effects of air pollution on dementia, cognitive function, and cognitive decline among adults conducted an evaluation of eleven prospective cohort studies25. Nearly all of these studies reported positive associations between long-term air pollution exposure and the incidence of dementia. However, these studies have notable limitations. For example, many of these studies did not model air pollution concentrations at a spatial resolution high enough to capture local emission variations. Given that local emissions have been associated with higher risk coefficients compared to urban background concentrations26,27, it is possible that investigations into urban background air pollution underestimate its health effects. Another shortfall in current evidence lies in the limited assessment of differential associations based on pollutant source. Due to their unique chemical compositions and toxicity, distinct PM sources may exert varying health effects. In one of few studies on source-specific air pollutants and dementia, Shi and colleagues recently found that long-term exposure to PM2.5 was significantly associated with higher rates of incident dementia and AD in a large Medicare cohort in the USA and that sulfate, black carbon, and organic matter related to traffic and fossil fuel combustion appeared to be key contributors to these associations28. Additionally, there is a lack of studies conducted in regions characterized by relatively low air pollution levels. Such low-exposure settings are also valuable for establishing air quality guideline values.
The primary objective of this study was to evaluate the relationship between total and source-specific concentrations of ambient particulate air pollution, modeled with high spatial resolution, and the incidence of dementia in an area characterized by low levels of air pollution exposure.
Material and methods
Study population
The study population comprised individuals enrolled in the Västerbotten Intervention Program (VIP) cohort between January 1, 1990, and December 31, 2014. VIP is a comprehensive program that extends invitations to all residents of Västerbotten county, including those in Umeå municipality, to undergo health assessments upon reaching specific age milestones, including 40, 50, and 60 years (and occasionally 30 years during certain years)29,30. These screenings were initiated with the aim of identifying individuals at elevated risk of cardiovascular disease and diabetes through a combination of clinical examinations and interviews covering various risk factors, such as socioeconomic status, education, dietary habits, and physical activity. Prior to their enrollment in VIP, all participants provided informed consent.
To date, over 100,000 individuals have actively participated in the VIP program. Although participation rates have fluctuated between 48 and 67%, they remained consistently high at 66–67% from 1995 to 2005. An analysis conducted in 1998 to assess dropout rates indicated minimal social selection bias. A translated version of the questionnaire is available in the Supplementary Material. For the purpose of this study, residential address histories were acquired from Statistics Sweden records, utilizing the personal identification numbers of each cohort participant. Subsequently, these residential addresses were subjected to geocoding, with an automated process matching them against the Swedish Map** Cadastral and Land Registration Authority Databases. In cases of inconsistencies or inaccuracies, manual verification and correction of addresses were performed, ensuring the assignment of accurate geographical coordinates. Cohort members from the VIP program were considered for analysis until the time of permanent emigration from the study area, the occurrence of death, or the conclusion of the study period.
Outcome assessment
We identified cases of dementia using International Classification of Diseases, Tenth Revision (ICD-10) codes F01 and G30. Furthermore, individuals were classified as dementia cases from the commencement of medication treatment (coded as per the Anatomical Therapeutic Chemical code N06D), which was obtained from the Swedish Prescribed Drug Register. Incidence of hospitalization due to dementia was ascertained by cross-referencing participants’ personal identification numbers with the National Patient Register maintained by the Swedish National Board of Health and Welfare (https://www.socialstyrelsen.se/en/statistics-and-data/registers/). Additionally, individuals who neither received a prior dementia diagnosis nor were prescribed dementia medication were included as incident cases if they succumbed to dementia-related mortality. Data on the specific causes of mortality were extracted from the Cause of Death Register of the Swedish National Board of Health and Welfare, again utilizing each participant's personal identification number.
To ensure the exclusion of prevalent cases of dementia, which included those who received a dementia diagnosis within five years before recruitment or were prescribed dementia medication one year before recruitment, we employed strict criteria. Due to the availability of dispensed medication data starting only from 2005 onwards, the follow-up period was initiated in 2006 and concluded in 2015.
Exposure assessment
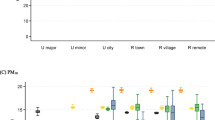
The Västerbotten county, situated in Northern Sweden, experiences notably low air pollution levels when viewed from an international perspective, particularly in terms of contributions from long-range sources (Fig. 1).
A comprehensive description of the dispersion model employed for exposure assessment has been previously documented31. In brief, emission inventories for the years 2000 and 2011 served as the foundation for Gaussian dispersion model simulations of particulate matter concentrations, specifically targeting PM10 and PM2.5. While the modeling occurred on an hourly basis, the results were consolidated into yearly average concentrations for our analyses. To obtain annual average concentrations for years falling between the modeled years (2000 and 2011), linear interpolation was utilized, assuming gradual emission changes over the intervening years. These interpolated values were also adjusted by a meteorological index, derived from continuous dispersion simulations conducted over a limited geographical area throughout the entire period (2000–2011). This adjustment accounted for year-to-year meteorological variations. For 2012 and 2013, concentrations were extrapolated following the same approach, assuming no significant alterations in emissions since 2011. To address pronounced spatial concentration variations near roads and in proximity to chimneys associated with residential stoves and boilers, a quadtree receptor grid was employed, achieving a spatial resolution as fine as 35 m × 35 m. For inner-city streets flanked by buildings on one or both sides, an additional concentration component was simulated using the Operational Street Pollution Model (OSPM)32. PM-exhaust attributable to road traffic, which considers different vehicle types, speeds, and driving conditions, was computed based on the Handbook on Emission Factors for Road Traffic, version 3.133. Non-exhaust emissions stemming from traffic-related PM primarily encompass particles from road wear, with a minor contribution from brake and tire wear34,35,36. The contribution of non-exhaust emissions to the yearly mean concentrations was estimated under the assumption of a spatial distribution identical to that of exhaust emissions. The exhaust-to-non-exhaust emissions ratio was estimated for each modeling year using methodologies detailed in Omstedt et al.36. Emissions for small-scale residential heating (PM-wood burning) were derived from a comprehensive inventory encompassing individual privately owned stoves and boilers, supplemented with data from chimney sweepers and insights into wood-burning habits obtained through interviews31. Industrial and energy production facilities were represented as point sources within the model. Emissions from ship** were also incorporated, with a methodology akin to that described by Jalkanen et al.35.
Annual mean concentrations of long-range transported PM10 and PM2.5 were predicated on measurements from regional background stations. Given the demonstrated small-scale spatial variation between regional background sites37, no additional adjustments were applied within the assessment area. Total annual concentrations of PM were computed as the sum of these regional background measurements and locally modeled concentrations. In a previous study, the validation of modelled concentrations against measurements from an urban background site in the study area yielded a 17% average difference across six different years for PM2.5, whereas the corresponding number was 49% for measurements taken at a traffic site in the study area31. For PM10, the corresponding average differences were 10% for the urban background site and 2% for the traffic site31.
Ultimately, the resultant concentrations encompassed total PM10, total PM2.5, and local, source-specific PM concentrations (PM10-Traffic, PM2.5-Exhaust, PM2.5-Wood burning) were estimated for each study participant's residential address. Changes in address during the exposure assessment period (2000–2013) were duly accounted for. PM10-Traffic encompassed particles originating from exhaust sources (primarily PM2.5) as well as non-exhaust particles stemming from road wear, brake, and tire wear (primarily PM2.5–10).
Confounders
To address potential confounding factors, various covariate data were gathered, including calendar year, gender, smoking status (current, former, never smoker), alcohol consumption (daily, weekly, seldom, never), physical activity during leisure time (sedentary, moderate, intermediate, or vigorous), marital status (single, married or cohabiting, data missing), educational attainment (primary school or lower, up to secondary school or equivalent, university degree or higher, data missing), and employment status (employed, unemployed/not gainfully employed, retired, data missing). Furthermore, the socioeconomic status at the area level was estimated based on each cohort member's mean neighborhood income. This calculation utilized individual incomes of individuals of working age for the calendar year 1994, as reported by Small Areas for Market Statistics (SAMS).
Statistical methods
We employed Cox-proportional hazard models to estimate hazard ratios (HR) for dementia in association with various PM sources (total PM10, total PM2.5, PM10-Traffic, PM2.5-Exhaust, and PM2.5-Wood burning). Age served as the underlying time variable for the baseline hazard. The regression model was adjusted for calendar year, baseline individual risk factors, and area-level socioeconomic status, with consistent covariate inclusion across all analyses. PM associations were evaluated using two exposure windows: a moving average over 1–5 years before the event (lag 1–5) and 6–10 years before the event (lag 6–10). To be included, annual mean air pollution concentrations were required to be available for at least 80% of the time window. Both single- and two-pollutant models were employed. HRs and their corresponding 95% CIs were presented in tertiles of PM. Due to low statistical power, it was not possible to evaluate concentration-responses at a great many points, hence the choice of tertiles for exposure rather than, for example, quartiles or cubic splines.
Ethical approval
The study underwent review and received approval from the Ethical Review Board in Umeå (reference 2015/16-31Ö). All methods were carried out in accordance with relevant guidelines and regulations.
Results
Participant characteristics
The study encompassed 70,057 individuals, with 53% being women, and a median age of 40 years at recruitment. Regarding individual risk factors, 20% were current smokers, 28% were former smokers, and 57% consumed alcohol on a weekly basis (Table 1). Furthermore, 40% did not engage in exercise that required training attire during their leisure time. In terms of socioeconomic status, 21% of cohort members were not in a domestic partnership, 38% had an education level of primary school or lower, and 6% were unemployed. The distribution of these individual risk factors is presented in Table 1, further stratified by estimated PM concentrations, using PM10-Traffic as an illustration. In the highest tertile of PM10-Traffic, there was a slightly higher proportion of female participants, individuals who had never smoked, those who engaged in regular physical activity during leisure time, individuals with higher education levels, and gainfully employed individuals. Over a total of 884,847 person-years of follow-up, we identified 409 incident cases of dementia.
Particle concentrations
Mean lag 1–5 concentrations of total PM10, total PM2.5, PM10-Traffic, PM2.5-Exhaust and PM2.5-Wood burning were 9.50, 5.61, 0.51, 0.10 and 0.78 µg/m3 during follow-up (Table 2). Overall, lag 6–10 concentrations were similar to lag 1–5 concentrations but lag 6–10 concentrations for total PM2.5 were slightly higher due to a decreasing trend in total PM concentrations during the study period. Total PM2.5 concentrations were correlated with PM10-Traffic and PM2.5-Wood burning (r = 0.3 and r = 0.4, respectively, for lag 1–5); however, the correlation between PM10-Traffic and PM2.5-Wood burning was low (r = 0.06 for lag 1–5).
Dementia incidence
Single-pollutant models
For concentrations of total PM10 for lag 1–5, the risk estimates for dementia incidence were 17% (95% CI −39 to 11%) and 18% (95% CI − 39 to 11%) lower in the second and third exposure tertiles, respectively, compared to the first (Fig. 2A). These risk reductions were lower compared to lag 6–10 for total PM10. For analyses on total PM2.5, decreased risks were also observed. Estimates for both total PM10 and total PM2.5 were low in precision.
Concerning PM2.5-Exhaust, the risk estimates for incident dementia were 33% (95% CI − 2 to 79%) and 11% (− 20 to 52%) higher in the second exposure tertile compared to the first for lag 1–5 and lag 6–10, respectively (Fig. 2A). No risk increases were observed in the third tertile, however. Risk estimates for PM10-Traffic were very similar to those for PM2.5-Exhaust. Regarding PM2.5-Wood burning, risk estimates for lag 1–5 were 33% (95% CI − 49 to − 11%) and 24% (95% CI − 46 to 6%) lower in the second and third exposure tertiles, respectively, compared to the first. For lag 6–10 of PM2.5-Wood burning, the risk estimate was 9% (95% CI − 18 to 44%) higher in the second tertile but 20% (95% CI − 43 to 13%) lower in the third tertile compared to the first.
Two-pollutant model
In the two-pollutant model including both lag 1–5 PM10-Traffic and PM2.5-Wood burning, the risk of incident dementia was 43% (95% CI 9–94%) higher in the second exposure tertile but only 16% (95% CI − 15 to 57%) higher in the third tertile compared to the first (Fig. 2B). In relation to lag 6–10 exposure, the single-pollutant model estimates did not significantly change following the simultaneous adjustment of PM10-Traffic and PM2.5-Wood burning.
Discussion
Main findings
While there were indications of a potential association between dementia risk and particulate matter from traffic sources (PM10-Traffic and PM2.5-Exhaust), it appeared that the relationship was not strictly linear. Conversely, concerning total PM10, total PM2.5, and PM2.5-Wood burning, there were hints of reduced dementia risk in the higher exposure tertiles, albeit with limited precision. Conducting research in low exposure settings is crucial for establishing air quality guideline values, making this study a significant addition to our existing knowledge despite the somewhat inconsistent findings.
Evidence on total particulate matter and dementia incidence
While The Lancet Commission on Dementia Prevention, Intervention, and Care report indicated that 2% of dementia cases could be attributed to air pollution5, the evidence regarding the association between dementia incidence and total PM2.5 exposure has yielded mixed results. For instance, a study using the Women's Health Initiative Memory Study (WHIMS) cohort found no significant link between annual PM2.5 exposure (IQR 3.9 µg/m3) and dementia incidence38, consistent with the findings of our present study. The Rotterdam study also failed to detect a clear association between air pollution exposure (modelled levels of PM10, PM2.5, and NO2) and the risk of dementia or cognitive decline39. However, a longitudinal, population-based study employing data from the Swedish National Study on Aging and Care in Kungsholmen demonstrated a 54% increased risk of dementia per inter-quartile range difference (IQR 0.88 µg/m3) of PM2.5, although most of this association could be attributed to cardiovascular comorbidities27. Another study involving seven European cohorts in low exposure settings found no association between air pollution and dementia mortality40.
On the other hand, most studies have reported positive associations. A recent meta-analysis, where two new cohorts in China were included, revealed that dementia risk increased with exposure to PM2.5, PM10, NO2, and nitrogen oxides (NOX)41. In the Betula study, which was conducted partly in the same study area as our present study, we observed that each 1 µg/m3 difference in annual mean PM2.5 concentration was associated with a hazard ratio of 1.23 (95% CI 1.01–1.50) for dementia42. The Rome Longitudinal Study, utilizing a large administrative cohort, reported mixed results, with exposure to NOX, NO2, PM2.5, and PM10 showing negative associations with AD but positive associations with vascular dementia43. Another study reported a positive association between PM2.5 exposure and dementia incidence, albeit not statistically significant44. In contrast, a different study within the same cohort reported contrasting results and found a 92% increased risk of dementia in the fourth quartile of PM2.5 exposure compared to the first23. A Canadian study discovered a positive association, citing a 4% increased risk for every IQR (3.4 µg/m3) increase in PM2.5 exposure45. In a London-based study, a 6% increased incidence of all-cause dementia was identified for each IQR (0.95 µg/m3) increase in PM2.5 exposure26. Additionally, an IQR increase in baseline PM2.5 exposure was associated with a 9% increase in dementia mortality in the United States Veterans Health Administration (VA) study67. Additionally, we lacked information on occupational factors, which may be related to dementia risk68. Exploring factors like shift work would have been particularly interesting69,70.
Our study’s exposure window was limited to the last ten years, and we could not investigate the potential role of earlier-life exposure. Finally, our findings may not be generalizable to settings with high concentrations of air pollution since our study was conducted in an area where, from a global perspective, total air pollution concentrations are relatively low. Nevertheless, concentrations of locally produced air pollution in this study area are quite similar to those in other cities in Sweden and other Nordic countries, which may support the generalizability of our results to such settings.
Future research
Further research on the association between air pollution, particularly PM and its locally produced sources71, and dementia is required, as current evidence on this environmental risk factor is somewhat inconsistent. Such studies are especially lacking in low- and middle-income countries where air pollution concentrations, sources and, thereby, its composition, can differ substantially from that of high-income countries. To date, there are furthermore very few studies on short-term exposure to air pollution and outcomes related to brain function, although short-term pollution-attributable decrements in default mode network functional connectivity was recently observed in a human exposure study12. Life course air pollution exposure in relation to cognitive health is another under-studied area, where more studies are needed72.
In this unique low-exposure setting located in Northern Sweden, our present study contributes valuable insights to the ongoing discourse regarding the potential link between long-term exposure to local sources of particulate air pollution and the risk of dementia incidence. Our findings, while shedding light on this complex issue, do not provide definitive evidence of a clear linear association in this specific context.
The significance of our study lies in its meticulous examination of a region characterized by relatively low levels of air pollution, especially when viewed from an international perspective. This distinct setting allows us to explore the impact of air pollution in an environment where total air pollution concentrations are notably lower than those observed in many other parts of the world.
However, despite the rigorous methodology employed and the wealth of data collected, our results do not unequivocally establish a direct link between long-term exposure to local sources of particulate air pollution and an increased risk of develo** dementia. While some previous research has suggested such associations, our study, conducted in an area with low air pollution levels, does not provide robust confirmation of these claims. Future studies should, furthermore, aim to investigate potential non-linearities of such associations, which would require larger statistical power than in the present study. Indeed, some findings indicate that exposure to outdoor air pollutants, particularly PM10, may non-linearly increase the risk of mild cognitive impairment progressing to dementia once a certain ambient air concentration threshold is surpassed73.
It's important to recognize that the relationship between air pollution and dementia is intricate and multifaceted. Our study adds to the growing body of literature on this topic but underscores the need for further investigation in diverse geographic settings and with various levels of air pollution exposure. The absence of a definitive link in this low-exposure context highlights the complexity of this research area and the potential influence of local factors on the observed outcomes.
Conclusion
In conclusion, our study underscores the importance of considering the specific environmental context when assessing the impact of air pollution on health outcomes. While it does not provide conclusive evidence of a direct connection between local sources of particulate air pollution and dementia incidence in this low-exposure setting, it contributes valuable data to the broader scientific understanding of this critical issue. Future research in different environments will be instrumental in unraveling the complex relationship between air quality and cognitive disorders.
Data availability
The data that support the findings of this study are available from Region Västerbotten but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Region Västerbotten.
References
Béjot, Y. et al. Impact of the ageing population on the burden of stroke: The dijon stroke registry. Neuroepidemiology 52, 78–85 (2019).
Feigin, V. L. et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 459–480 (2019).
Lobo, A. et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology 54, S4 (2000).
Religa, D. et al. SveDem, the Swedish dementia registry–A tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PloS one 10, e0116538 (2015).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396, 413–446 (2020).
Genc, S., Zadeoglulari, Z., Fuss, S. H. & Genc, K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012, 1–23 (2012).
Calderón-Garcidueñas, L. et al. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 68, 117–127. https://doi.org/10.1016/j.bandc.2008.04.008 (2008).
Calderón-Garcidueñas, L. et al. Air pollution and brain damage. Toxicol. Pathol. 30, 373–389 (2002).
Calderon-Garciduenas, L. et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol. Pathol. 31, 524–538 (2003).
Campbell, A. et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology 26, 133–140 (2005).
Kleinman, M. et al. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol. Lett. 178, 127–130 (2008).
Troubat, R. et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 53, 151–171 (2021).
Gawryluk, J. R., Palombo, D. J., Curran, J., Parker, A. & Carlsten, C. Brief diesel exhaust exposure acutely impairs functional brain connectivity in humans: A randomized controlled crossover study. Environ. Health 22, 1–7 (2023).
Costa, L. G. et al. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 210, 107523 (2020).
Block, M. L. & Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32, 506–516 (2009).
Kulick, E. R. et al. Long-term exposure to ambient air pollution, APOE-ε4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environ. Int. 136, 105440 (2020).
Power, M. C., Adar, S. D., Yanosky, J. D. & Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. Neurotoxicology 56, 235–253 (2016).
Tzivian, L. et al. Long-term air pollution and traffic noise exposures and cognitive function: A cross-sectional analysis of the Heinz Nixdorf recall study. J. Toxicol. Environ. Health Part A 79, 1057–1069 (2016).
Cristaldi, A. et al. Possible association between PM2.5 and neurodegenerative diseases: A systematic review. Environ. Res. 208, 112581 (2022).
Calderón-Garcidueñas, L. et al. Interactive and additive influences of Gender, BMI and apolipoprotein 4 on cognition in children chronically exposed to high concentrations of PM2.5 and ozone. APOE 4 females are at highest risk in Mexico City. Environ. Res. 150, 411–422. https://doi.org/10.1016/j.envres.2016.06.026 (2016).
Chen, H. et al. Exposure to ambient air pollution and the incidence of dementia: A population-based cohort study. Environ. Int. 108, 271–277. https://doi.org/10.1016/j.envint.2017.08.020 (2017).
Ran, J. et al. Long-term exposure to fine particulate matter and dementia incidence: A cohort study in Hong Kong. Environ. Pollut. 271, 116303. https://doi.org/10.1016/j.envpol.2020.116303 (2021).
Cacciottolo, M. et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl. Psychiatry 7, e1022–e1022 (2017).
Park, C. et al. Associations between long-term air pollution exposure and plasma amyloid beta in very old adults. Alzheimer’s Dement. 17, e054700 (2021).
Delgado-Saborit, J. M. et al. A critical review of the epidemiological evidence of effects of air pollution on dementia, cognitive function and cognitive decline in adult population. Sci. Tot. Environ. 757, 143734 (2021).
Carey, I. M. et al. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ open 8, e022404 (2018).
Grande, G., Ljungman, P. L., Eneroth, K., Bellander, T. & Rizzuto, D. Association between cardiovascular disease and long-term exposure to air pollution with the risk of Dementia. JAMA Neurol. 77(7), 801–809 (2020).
Shi, L. et al. Incident dementia and long-term exposure to constituents of fine particle air pollution: A national cohort study in the United States. Proc. Natl. Acad. Sci. 120, e2211282119 (2023).
Norberg, M. et al. Community participation and sustainability – evidence over 25 years in the Västerbotten Intervention Programme. Glob Health Action https://doi.org/10.3402/gha.v5i0.19166 (2012).
Norberg, M., Wall, S., Boman, K. & Weinehall, L. The Västerbotten Intervention Programme: Background, design and implications. Glob. Health Action https://doi.org/10.3402/gha.v3i0.4643 (2010).
Segersson, D. et al. Health impact of PM10, PM2.5 and black carbon exposure due to different source sectors in Stockholm, Gothenburg and Umea, Sweden. Int. J. Environ. Res. Public Health 14, 742 (2017).
Berkowicz, R. OSPM-A parameterised street pollution model. Environ. Monit. Assess. 65, 323–331 (2000).
Hausberger, S. Emission factors from the model PHEM for the HBEFA version 3 (2009).
Denby, B. R. et al. A coupled road dust and surface moisture model to predict non-exhaust road traffic induced particle emissions (NORTRIP). Part 2: Surface moisture and salt impact modelling. Atmos. Environ. 81, 485–503 (2013).
Jalkanen, J.-P. et al. Extension of an assessment model of ship traffic exhaust emissions for particulate matter and carbon monoxide. Atmos. Chem. Phys. 12, 2641–2659 (2012).
Omstedt, G., Bringfelt, B. & Johansson, C. A model for vehicle-induced non-tailpipe emissions of particles along Swedish roads. Atmos. Environ. 39, 6088–6097. https://doi.org/10.1016/j.atmosenv.2005.06.037 (2005).
Forsberg, B. et al. Comparative health impact assessment of local and regional particulate air pollutants in Scandinavia. AMBIO J. Hum. Environ. 34, 11–19 (2005).
Chen, J. -C. et al. Particulate air pollutants, brain structure, and neurocognitive disorders in older women. Res. Rep. Health Eff. Inst. 2017 (2017).
de Crom, T. O. et al. Air pollution and the risk of Dementia: The rotterdam study. J. Alzheimer’s Dis. 91, 1–11 (2022).
Andersen, Z. J. et al. Long-term exposure to air pollution and mortality from dementia, psychiatric disorders, and suicide in a large pooled European cohort: ELAPSE study. Environ. Int. 170, 107581 (2022).
Tang, J. et al. Association of air pollution with dementia: A systematic review with meta-analysis including new cohort data from China. Environ. Res. 223, 115048. https://doi.org/10.1016/j.envres.2022.115048 (2023).
Andersson, J. et al. PM 2.5 and Dementia in a low exposure setting: The influence of odor identification ability and APOE. J. Alzheimer’s Dis. 92, 1–11 (2023).
Cerza, F. et al. Long-term exposure to air pollution and hospitalization for dementia in the Rome longitudinal study. Environ. Health 18, 72 (2019).
Ilango, S. D. et al. The role of cardiovascular disease in the relationship between air pollution and incident dementia: A population-based cohort study. Int. J. Epidemiol. 49, 36–44 (2020).
Chen, H. et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 389, 718–726 (2017).
Bowe, B., **e, Y., Yan, Y. & Al-Aly, Z. Burden of cause-specific mortality associated with PM2.5 air pollution in the United States. JAMA Netw. Open 2, e1915834–e1915834 (2019).
Mortamais, M. et al. Long-term exposure to ambient air pollution and risk of dementia: Results of the prospective Three-City Study. Environ. Int. 148, 106376 (2021).
Shi, L. et al. A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat. Commun. 12, 6754 (2021).
Segersson, D., Johansson, C. & Forsberg, B. Near-source risk functions for particulate matter are critical when assessing the health benefits of local abatement strategies. Int. J. Environ. Res. Public Health 18, 6847 (2021).
Herich, H. & Hueglin, C. Residential wood burning: A major source of fine particulate matter in Alpine Valleys in Central Europe. Urban Air Qual. Eur. 26, 123–140 (2013).
Oudin, A., Segersson, D., Adolfsson, R. & Forsberg, B. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PloS one 13, e0198283 (2018).
Oudin, A. et al. Traffic-related air pollution and dementia incidence in Northern Sweden: A longitudinal study. Environ. Health Perspect. 124, 306. https://doi.org/10.1289/ehp.1408322 (2016).
Andersson, J., Oudin, A., Nordin, S., Forsberg, B. & Nordin, M. PM2.5 exposure and olfactory functions. Int. J. Environ. Health Res. 32, 2484–2495 (2022).
Oudin, A. et al. Traffic-related air pollution as a risk factor for Dementia: No clear modifying effects of APOE ɛ4 in the betula cohort. J. Alzheimer’s Dis. 71, 1–8 (2019).
González-Maciel, A., Reynoso-Robles, R., Torres-Jardon, R., Mukherjee, P. S. & Calderón-Garcidueñas, L. Combustion-derived nanoparticles in key brain target cells and organelles in young urbanites: Culprit hidden in plain sight in Alzheimer’s disease development. J. Alzheimer’s Dis. 59, 189–208 (2017).
Lindroth, M., Lundqvist, R., Lilja, M. & Eliasson, M. Cardiovascular risk factors differ between rural and urban Sweden: The 2009 Northern Sweden MONICA cohort. BMC public health 14, 1–8 (2014).
Jonsson, F., Goicolea, I. & San Sebastian, M. Rural–urban differences in health among youth in northern Sweden: An outcome-wide epidemiological approach. Int. J. Circumpolar Health 78, 1640015 (2019).
Neovius, M. & Rasmussen, F. Place of residence and obesity in 1,578,694 young Swedish men between 1969 and 2005. Obesity 16, 671–676 (2008).
Sjöberg, A. et al. Overweight and obesity in a representative sample of schoolchildren–exploring the urban–rural gradient in Sweden. Obes. Rev. 12, 305–314 (2011).
Maccora, J., Peters, R. & Anstey, K. J. What does (low) education mean in terms of dementia risk? A systematic review and meta-analysis highlighting inconsistency in measuring and operationalising education. SSM-Popul. Health 12, 100654 (2020).
Grøholt, E. K., Stigum, H., Nordhagen, R. & Köhler, L. Health service utilization in the Nordic countries in 1996: Influence of socio-economic factors among children with and without chronic health conditions. Eur. J. Public Health 13, 30–37 (2003).
Flanagan, E., Stroh, E., Oudin, A. & Malmqvist, E. Connecting air pollution exposure to socioeconomic status: A cross-sectional study on environmental injustice among pregnant women in Scania, Sweden. Int. J. Environ. Res. Public Health 16, 5116 (2019).
Hajat, A., Hsia, C. & O’Neill, M. S. Socioeconomic disparities and air pollution exposure: A global review. Curr. Environ. Health Rep. 2, 440–450 (2015).
Hietikko, R. et al. Diurnal variation of nanocluster aerosol concentrations and emission factors in a street canyon. Atmos. Environ. 189, 98–106 (2018).
Teinilä, K. et al. Characterization of particle sources and comparison of different particle metrics in an urban detached housing area, Finland. Atmos. Environ. 272, 118939 (2022).
Rizzuto, D. et al. Detection of dementia cases in two Swedish health registers: A validation study. J. Alzheimer’s Dis. 61, 1301–1310 (2018).
Andersson, J. et al. Road traffic noise, air pollution, and risk of dementia–results from the Betula project. Environ. Res. 166, 334–339 (2018).
Huang, L.-Y. et al. Association of occupational factors and dementia or cognitive impairment: A systematic review and meta-analysis. J. Alzheimer’s Dis. 78, 217–227 (2020).
Leso, V., Caturano, A., Vetrani, I. & Iavicoli, I. Shift or night shift work and dementia risk: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 25, 222–232 (2021).
Jørgensen, J. T. et al. Shift work and incidence of dementia: A Danish nurse cohort study. Alzheimer’s Dement. 16, 1268–1279 (2020).
Chandra, M. et al. Air pollution and cognitive impairment across the life course in humans: A systematic review with specific focus on income level of study area. Int. J. Environ. Res. Public Health 19, 1405 (2022).
Russ, T. C. et al. Life course air pollution exposure and cognitive decline: Modelled historical air pollution data and the Lothian birth cohort 1936. J. Alzheimer’s Dis. 79, 1063–1074 (2021).
Urbano, T. et al. Particulate matter exposure from motorized traffic and risk of conversion from mild cognitive impairment to dementia: An Italian prospective cohort study. Environ. Res. 222, 115425. https://doi.org/10.1016/j.envres.2023.115425 (2023).
Acknowledgements
We extend our sincere gratitude to the individuals who participated in the Västerbotten Intervention Programme, without whom this research would not have been possible. This study received essential support from various sources, including Formas, DNR 2017-00898 (How is our health affected by particles from wood burning?). It was also funded by the European Union’s Horizon 2020 Call under the project titled “TUBE: Transport derived Ultrafines and the Brain Effects,” with the project number 814978-2. Additionally, our research benefited from the ADAIR project, which received grant funding under the reference #JPND2019-466-037. This project is part of the European Union Joint Programme—Neurodegenerative Disease Research (JPND), a collaborative effort involving multiple funding organizations from different countries across Europe. You can learn more about JPND at www.jpnd.eu. Furthermore, we would like to acknowledge the support of the following funding organizations participating in JPND, whose contributions were instrumental in advancing our research: Ministry of Education, Youth and Sports, Czech Republic; Academy of Finland, Finland; National Research, Development and Innovation Office, Hungary; Ministry of Education, Universities and Research, Italy; ZonMW – The Netherlands Organisation for Health Research and Development, The Netherlands; Swedish Research Council, Sweden. Finally, Dr. Töpi Rönkkö's involvement in this research was made possible through a grant from the Academy of Finland Flagship Programme, with the grant number 337551. Their dedicated contributions significantly enriched our study and its findings.
Funding
Open access funding provided by Umea University.
Author information
Authors and Affiliations
Contributions
A.O. was responsible for Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Interpretation, Project administration, Resources and Writing—original draft, review and editing. W.R. contributed to the Investigation, Methodology and Writing—original draft, review and editing. E.F. participated in Writing—original draft, review and editing. DS was responsible for Formal analysis, Writing—review and editing and creating Fig. 1. P.J., K.M.K., T.R., T.S. and J.T. contributed through Funding acquisition and Writing—original draft. R.G. and A.M. helped with Writing—original draft. J.S. was responsible for Formal analysis, Investigation, Methodology, Writing—original draft, review and editing as well as creating Fig. 2A,B. All authors have seen and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oudin, A., Raza, W., Flanagan, E. et al. Exposure to source-specific air pollution in residential areas and its association with dementia incidence: a cohort study in Northern Sweden. Sci Rep 14, 15521 (2024). https://doi.org/10.1038/s41598-024-66166-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66166-y
- Springer Nature Limited