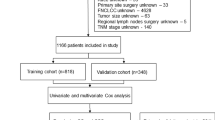
Abstract
With the aging world population, the incidence of soft tissue sarcoma (STS) in the elderly gradually increases and the prognosis is poor. The primary goal of this research was to analyze the relevant risk factors affecting the postoperative overall survival in elderly STS patients and to provide some guidance and assistance in clinical treatment. The study included 2,353 elderly STS patients from the Surveillance, Epidemiology, and End Results database. To find independent predictive variables, we employed the Cox proportional risk regression model. R software was used to develop and validate the nomogram model to predict postoperative overall survival. The performance and practical value of the nomogram were evaluated using calibration curves, the area under the curve, and decision curve analysis. Age, tumor primary site, disease stage, tumor size, tumor grade, N stage, and marital status, are the risk variables of postoperative overall survival, and the prognostic model was constructed on this basis. In the two sets, both calibration curves and receiver operating characteristic curves showed that the nomogram had high predictive accuracy and discriminative power, while decision curve analysis demonstrated that the model had good clinical usefulness. A predictive nomogram was designed and tested to evaluate postoperative overall survival in elderly STS patients. The nomogram allows clinical practitioners to more accurately evaluate the prognosis of individual patients, facilitates the progress of individualized treatment, and provides clinical guidance.
Similar content being viewed by others
Introduction
Soft tissue sarcoma (STS) is a rare mesenchymal-derived tumor that most often occurs in the extremities. It is subdivided into about 75 distinct subgroups with distinct biology, molecular abnormalities, and therapy responses. Due to the rarity of such cancers and their numerous varieties, no large-scale data exist to guide treatment, necessitating a multidisciplinary and customized approach1,2. The incidence of STS in senior individuals has been growing in recent years, as the world ages3,4. Recent research indicates that STS instances occur in people 65 years of age, with the frequency of sarcoma increasing with age, and there is no conclusive idea regarding the best complete treatment for senior STS patients5,6. The current studies overall, these studies suggest that surgery in combination with radiotherapy remains the best means of controlling STS today compared to other treatments. Although increasing age shows a negative correlation with STS-specific survival, the prognosis for elderly patients with STS is favorable when older patients of appropriate age are selected to undergo extensive STS resection7,8,9. According to a study monitoring the epidemiology of STS, the analysis showed that fewer patients ≥ 85.5 years of age underwent surgical treatment10. Furthermore, recent studies suggest that STS cases gradually rise in patients 65 years and older. Therefore, it is important to study the clinical-related prognostic factors in STS elderly groups.
Given the relatively rare incidence of STS, clinical studies for STS are typically difficult, necessitating a more rigorous design that includes patient screening and therapy assignment. The nomogram combines and illustrates several important prognostic factors, is a reliable and valid tool for quantifying individual risk, and performs well in predicting survival in various cancers. In previous studies, nomograms for STS in specific tumor primary locations or STS of specific histological types (e.g. liposarcoma, fibrosarcoma) have been developed11,3). In both the training and validation sets, DCA demonstrated the good clinical usefulness of the nomogram in predicting postoperative OS in elderly patients with STS, as shown in Fig. 4.
Risk classification system
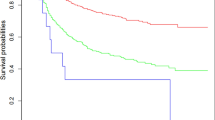
The optimum cut-off point of the overall score based on OS was determined using X-tiles software, and all cases were classified into low risk (295), middle risk (295–340), and high risk (> 340) groups. Kaplan–Meier survival curves for each risk subgroup were plotted and log-rank tests were performed, which showed differences in OS among patients with different risk levels (P < 0.001) (Fig. 5). Patients in the high risk group had a worse outcome than those in the low risk group in the training and validation set. It suggests that the nomogram-based risk classification system seems to have a high predictive ability for the OS.
Discussion
In this study, a clinical prediction model was constructed by the SEER database to predict postoperative OS in elderly patients with STS. The nomogram we created, as shown in Fig. 3, can accurately predict OS at 1, 3, and 5 years. This is the first study to use extensive and diverse case data to build a predictive nomogram model for elderly postoperative patients with STS. This prediction nomogram can easily predict patients’ prognosis, inform patients of the benefits of certain treatments, and have important implications for clinical decision-making.
It’s vital to keep in mind that not all groups of elderly adults with STS can be surgically treated. In the present results, the prognosis of elderly postoperative patients with STS > 82 years of age was worse compared to patients in the 73–82 years age group. It was similarly confirmed in previous single-center institutional studies that age is not a contraindication to surgery in elderly cancer patients with STS15,16,17. Buchner et al. prospectively studied 21 STS patients over the age of 70, and they discovered that even after surgery, these patients had a worse 5-year OS18. Lahat et al. analyzed 325 STS ≥ 65 years of age patients while selecting appropriate elderly patients among these individuals for surgery. They noted a decrease in bit survival after surgery in patients > 75 years of age. However, postoperative adverse effects and recovery time did not differ significantly between these STS age groups7. In another study it was also confirmed that surgery is safe and that reduced surgical use in the elderly may be an area of improved prognosis, while this study also noted a significant reduction in mortality in the elderly at 90 days after undergoing STS, suggesting that the postoperative 90-day period is key to the increased surgical risk affecting elderly patients with STS. Whether these factors influence surgical decision-making in elderly patients directly or indirectly19. Taken together, each of these studies concludes that despite the reduced OS in the elderly patient population after surgery compared to the younger patient population, surgery is still advisable for the relatively low perioperative complication rate and postoperative adverse effects in a subset of appropriate elderly patients with STS.
Whether tumors metastasize or not, disease stage and tumor size also have a different prognosis for elderly patients with STS after surgery. Elderly patients with STS who developed distant metastasis in this study had a poorer OS in comparison to elderly patients with localized tumors. The poorer prognosis after surgery in elderly patients with STS with tumor diameter size > 100 mm is an important disadvantage. Several studies to date have confirmed the prognostic factors associated with the diagnosis of STS in adults. In previous studies, the most common unfavorable prognostic factors for elderly postoperative patients with STS were found to be: (1) Site of tumor occurrence: including head, neck, and trunk, (2) different subtypes of tumors, (3) deeper tumor location, (4) residual tumor cells are still present at the surgical incision margin, (5) tumor size > 5 cm, and other factors20,21,22,23,24. Previous research has indicated that younger STS patients are more likely than older STS patients to develop tumors in a deeper position. Smaller tumor diameter and lower tumor grade were found to be independent risk variables for recurrence-free survival and OS in younger and older STS patients, even though older STS patients had larger tumors and a higher proportion of graded tumors25. Therefore, compared with younger patients with STS, older patients have relatively larger tumors, higher tumor stage, and grade, and are prone to distant metastases, causing some surgical difficulty and making surgical decisions difficult, which may also contribute to the poorer prognosis of older patients with STS.
Conclusion
We constructed and verified a predictive nomogram to estimate the personalized postoperative OS of elderly patients with STS. The nomogram allows clinical practitioners to more accurately evaluate the prognosis of individual patients, facilitates the progress of individualized treatment, and provides clinical guidance.
Data availability
The dataset from the SEER database that was generated and/or analyzed during the current study is available in the SEER dataset repository (https://seer.cancer.gov/). The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- STS :
-
Soft tissue sarcoma
- OS :
-
Overall survival
- SEER :
-
Surveillance, epidemiology, and end results
- ROC :
-
Receiver operating characteristic curves
- AUC :
-
Area under the curve
- DCA :
-
Decision curve analysis
References
Ayodele, O. & Razak, A. Immunotherapy in soft-tissue sarcoma. Curr. Oncol. 27, 17–23 (2020).
Meyer, M. & Seetharam, M. First-line therapy for metastatic soft tissue sarcoma. Curr. Treat. Opt. Oncol. 20(1), 6 (2019).
Hoefkens, F., Dehandschutter, C., Somville, J., Meijnders, P. & Van Gestel, D. Soft tissue sarcoma of the extremities: Pending questions on surgery and radiotherapy. Radiat. Oncol. 11(1), 136 (2016).
Georgios-Ioannis, V. et al. Mid-term outcomes in the treatment of retroperitoneal sarcomas: A 12-year single-institution experience. Med. Glas. https://doi.org/10.17392/1498-22 (2022).
Graham, D. et al. Chemotherapy and survival in patients with primary high-grade extremity and trunk soft tissue sarcoma. Cancers 12(9), 2389 (2020).
Chowdhary, M. et al. Does the addition of chemotherapy to neoadjuvant radiotherapy impact survival in high-risk extremity/trunk soft-tissue sarcoma?. Cancer 125(21), 3801–3809 (2019).
Lahat, G. et al. Complete soft tissue sarcoma resection is a viable treatment option for select elderly patients. Ann. Surg. Oncol. 16(9), 2579–2586 (2009).
Toro, J. et al. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int. J Cancer 119(12), 2922–2930 (2006).
Boden, R. et al. Surgical management of soft tissue sarcoma in patients over 80 years. Eur. J. Surg. Oncol. 32(10), 1154–1158 (2006).
Al-Refaie, W. B. et al. Extremity soft tissue sarcoma care in the elderly: Insights into the generalizability of NCI cancer trials. Ann. Surg. Oncol. 17(7), 1732 (2010).
Ye, L. et al. Nomogram for predicting the overall survival and cancer-specific survival of patients with extremity liposarcoma: A population-based study. BMC cancer 20(1), 889 (2020).
Yang, F., **e, H. & Wang, Y. Prognostic nomogram and a risk classification system for predicting overall survival of elderly patients with fibrosarcoma: A population-based study. J. Oncol. 2021, 9984217 (2021).
Callegaro, D., Miceli, R., Mariani, L., Raut, C. & Gronchi, A. Soft tissue sarcoma nomograms and their incorporation into practice. Cancer 123(15), 2802–2820 (2017).
Callegaro, D. et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 17(5), 671–680 (2016).
Lahat, G., Lazar, A. & Lev, D. Sarcoma epidemiology and etiology: Potential environmental and genetic factors. Surg. Clin. N. Am. 88(3), 451–481 (2008).
Yoneda, Y. et al. Favorable outcome after complete resection in elderly soft tissue sarcoma patients: Japanese musculoskeletal oncology group study. Eur. J. Surg. 40(1), 49–54 (2014).
Osaka, S., Sugita, H., Osaka, E., Yoshida, Y. & Ryu, J. Surgical management of malignant soft tissue tumours in patients aged 65 years or older. J. Orthop. Surg. 11(1), 28–33 (2003).
Buchner, M., Bernd, L., Zahlten-Hinguranage, A. & Sabo, D. Primary malignant tumours of bone and soft tissue in the elderly. Eur. J. Surg. Oncol. 30(8), 877–883 (2004).
Gingrich, A. et al. Extremity soft tissue sarcoma in the elderly: Are we overtreating or undertreating this potentially vulnerable patient population?. J. Surg. Oncol. 119(8), 1087–1098 (2019).
Vraa, S., Keller, J., Nielsen, O. S., Jurik, A. G. & Jensen, O. M. Soft-tissue sarcoma of the thigh: Surgical margin influences local recurrence but not survival in 152 patients. Acta Orthop. Scand. 72(1), 72 (2001).
Engellau, J. et al. Improved prognostication in soft tissue sarcoma: independent information from vascular invasion, necrosis, growth pattern, and immunostaining using whole-tumor sections and tissue microarrays. Hum. Pathol. 36(9), 994–1002 (2005).
Zagars, G. et al. Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: Analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 57(3), 739–747 (2003).
Zagars, G. et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 97(10), 2530–2543 (2003).
Ogura, K., Higashi, T. & Kawai, A. Statistics of bone sarcoma in Japan: Report from the bone and soft tissue tumor registry in Japan. J. Orthop. Sci. 22(1), 133–143 (2017).
Kasper, B. et al. Prognostic factors in adolescents and young adults (AYA) with high risk soft tissue sarcoma (STS) treated by adjuvant chemotherapy: a study based on pooled European organisation for research and treatment of cancer (EORTC) clinical trials 62771 and 62931. Eur. J. Cancer 49(2), 449–456 (2013).
Funding
This study was funded by the **hua traditional Chinese Medicine Science and Technology Research Program Project (2024LC11) and the Health Science and Technology Plan Project of Fujian Province (No.2022QNA065).
Author information
Authors and Affiliations
Contributions
XM L and QS G conceived and designed the study. XM L and QS G performed the literature search. LJ Z and GC L generated the figures and tables. ZH H and XM L analyzed the data. XM L wrote the manuscript and QS G critically reviewed the manuscript. XM L and QS G supervised the research. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lv, X., Zhu, L., Lan, G. et al. A clinical tool to predict overall survival of elderly patients with soft tissue sarcoma after surgical resection. Sci Rep 14, 15098 (2024). https://doi.org/10.1038/s41598-024-65657-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65657-2
- Springer Nature Limited