Abstract
Background
Primary retroperitoneal sarcoma (RPS) comprises over 70 histologic subtypes, yet there are limited studies that have developed prognostic nomograms for RPS patients to predict overall survival (OS) and cancer-specific survival (CSS). The objective of this study was to construct prognostic nomograms for predicting OS and CSS in RPS patients.
Methods
We identified a total of 1166 RPS patients from the Surveillance, Epidemiology and End Results (SEER) database, and an additional 261 cases were collected from a tertiary cancer center. The study incorporated various clinicopathological and epidemiologic features as variables, and prediction windows for overall survival (OS) and cancer-specific survival (CSS) were set at 3, 5, and 7 years. Multivariable Cox models were utilized to develop the nomograms, and variable selection was performed using a backward procedure based on the Akaike Information Criterion. To evaluate the performance of the nomograms in terms of calibration and discrimination, we used calibration plots, coherence index, and area under the curve.
Findings
The study included 818 patients in the development cohort, 348 patients in the internal validation cohort, and 261 patients in the external validation cohort. The backward procedure selected the following variables: age, French Federation of Cancer Centers Sarcoma Group (FNCLCC) grade, pre-/postoperative chemotherapy, tumor size, primary site surgery, and tumor multifocality. The validation results demonstrated that the nomograms had good calibration and discrimination, with C-indices of 0.76 for OS and 0.81 for CSS. Calibration plots also showed good consistency between the predicted and actual survival rates. Furthermore, the areas under the time-dependent receiver operating characteristic curves for the 3-, 5-, and 7-year OS (0.84, 0.82, and 0.78, respectively) and CSS (0.88, 0.88, and 0.85, respectively) confirmed the accuracy of the nomograms.
Interpretation
Our study developed accurate nomograms to predict OS and CSS in patients with RPS. These nomograms have important clinical implications and can assist healthcare providers in making informed decisions regarding patient care and treatment options. They may also aid in patient counseling and stratification in clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Primary retroperitoneal sarcoma (RPS) is a rare malignancy, accounting for about 0.1% of adult cancers globally, and clinical estimates project an annual occurrence of 0.5 to 1 new case per 100,000 individuals [1]. RPS originates within the expansive retroperitoneal space, characterized by an absence of discernible symptoms during its initial stages. Clinical manifestations only become evident in the later stages when it encroaches upon or exerts pressure upon neighboring organs. At this juncture, the neoplasm often attains significant proportions and infiltrates adjacent structures, thus resulting in an unfavorable prognosis for the patient [2]. RPS comprises more than 70 different histologic subtypes [3], these types of pathology are further complicated by their variable presentation, behavior, and long-term outcomes, which emphasize the importance of accurate prognostic models to guide patient management. Surgical resection is the preferred treatment option for RPS, and it can significantly improve patient prognosis [4,5,6,7]. However, not all patients are suitable candidates for surgery due to factors such as metastasis or extensive tumor burden.
Due to the rarity of primary RPS and the diverse range of histological subtypes, predicting patient prognosis can be challenging. A nomogram is a valuable visual tool in clinical medicine. It provides personalized survival estimates based on individual patient characteristics, aiding doctors in understanding survival probabilities, assessing risk, and supporting treatment decisions. In clinical investigation, nomograms help identify suitable participants and enhance trial efficiency and reliability. They also facilitate patient and family understanding of survival probabilities and risks, improving trust and treatment plan adherence. In summary, nomograms significantly improve patient care and outcomes. Although several nomograms have been developed to predict the prognosis of RPS patients with surgical resection [15]. Age, tumor size, median household income, local population size, and degree of urbanization were converted to categorical variables. For OS, X-tile software divided age into three groups: ≤ 55 years, 56–78 years, and ≥ 79 years, tumor size was divided into two groups: ≤ 7.7 cm and > 7.7 cm; for CSS, age was divided into ≤ 54 years, 55–78 years and ≥ 79 years three groups, tumor size was divided into ≤ 10.4 cm and > 10.4 cm two groups. In both OS and CSS, the median household income of patients was divided into two groups: 0–54999 dollars and ≥ 55,000 dollars; population size and degree of urbanization in the patient’s place was divided into two groups [I: nonmetropolitan counties not adjacent to a metropolitan area, nonmetropolitan counties adjacent to a metropolitan area and counties in metropolitan areas of less than 250 thousand population; II: counties in metropolitan areas of 250,000 to 1 million population and counties in metropolitan areas greater than or equal to 1 million population]. Univariate and multivariate Cox regression analyses were performed based on the develo** set, variables with P < 0.05 in univariate Cox regression analysis were included in multivariate Cox regression analysis. To develop the two nomograms, we used multivariable Cox regression analysis using a backward procedure with Akaike information criterion (AIC) as the variables selection criterion for OS and CSS models [16]. Internal and external validation of the OS nomogram and internal validation of the CSS nomogram were performed, and concordance indices (C index) and calibration plots were used to measure discrimination and calibration ability. Furthermore, the accuracy of the nomograms for 3-, 5- and 7-year survival prediction were compared with the TNM staging system to demonstrate by using the area under (AUC) the receiver operating characteristic curve (ROC).
2.3 Statistical analysis
Statistical analysis was performed using R software, version 4.0.3 (R Foundation for Statistical Computing). Variable selection based on AIC was implemented using the MASS R package [17], potentially prognostic factors, age, sex, race and ethnicity, tumor size, histopathological type, FNCLCC grade, tumor multifocality, surgery of primary site, surgery of regional lymph nodes, pre-/postoperative chemotherapy, median household income, local population size and degree of urbanization, were all included. Multivariable Cox regression analysis was performed to calculate the hazard ratios, two-sided Wald test P values, and 95% confidence intervals of the selected variables and build the nomograms. A risk score of a single patient was calculated using the survival R package and the median was used as a cutoff value for high-risk and low-risk groups [18]. Statistical comparison of survival curves was using a log-rank test. The internal and external validation sets were applied in the model built using the develo** set. The bootstrap method was applied for 1000 iterations of computation.
3 Results
3.1 Patient characteristics
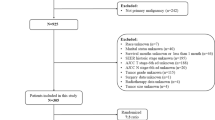
The baseline clinicopathological and epidemiological characteristics of the 1427 patients with RPS (mean [SD] age, 60.2 [15.0] years) were shown in Table 1, and the characteristics were well balanced between the develo** set and the validation set.
Among the study population, 619 (43.4%) patients were female, and 547 (38.3%) were male. The median age was 60 (range, 0–95) years. The median tumor size was 139.5 (range, minimum: 11, maximum: > 989) mm. The median follow-up time was 29 (range, 1–140) months. The median OS and CSS were 56.21 (IQR: 47.20–71.10) and 67.54 (IQR: 61.01–78.15) months, respectively. Patients diagnosed with liposarcoma, leiomyosarcoma, fibrosarcoma and other pathological types were 848 (59.4%), 397 (27.8%), 26 (1.9%) and 156 (10.9%) respectively. Race and ethnicity of patients were divided into 3 categories: White (906, 63.5%), Black (127, 8.9%), and others (394, 27.6%).
3.2 Variable selection and multivariable Cox analysis
Results of univariate and multivariate Cox regression analysis were displayed in Table 2, P < 0.05 was considered significant. Based on AIC, age, FNCLCC grade, chemotherapy, tumor size, primary site surgery, and tumor multifocality were identified as independent risk factors for OS and CSS. The results of the final OS and CSS multivariate Cox regression models were displayed in Table 3. The multivariable Cox analysis of the develo** set found that older age (56–78 years vs ≤ 55 years, hazard ratio [HR], 1.49; 95% confidence interval [CI] 1.14–1.96; P < 0.01; ≥ 79 years vs ≤ 55 years, HR, 3.00; 95% CI 2.06–4.38; P < 0.001), higher FNCLCC grade (grade II vs grade I, HR, 2.11; 95% CI 1.48–3.02; grade III vs grade I, HR, 3.95; 95% CI 2.85–5.49; both P < 0.001), pre-/postoperative of chemotherapy (yes vs no, HR, 2.01; 95% CI, 1.53–2.64; P < 0.001), tumor size > 7.7 cm (> 7.7 cm vs ≤ 7.7 cm, HR, 3.06; 95% CI 1.96–4.79; P < 0.001), no surgery operated on primary site (no vs yes, HR, 3.35; 95% CI 2.23–5.04; P < 0.001) were independent risk factors for worse OS, and older age (55–78 years vs ≤ 54 years, HR, 1.43; 95% CI 1.03–1.98; P < 0.05; ≥ 79 years vs ≤ 54 years, HR, 2.54; 95% CI 1.54–4.19; P < 0.001), higher FNCLCC grade (grade II vs grade I, HR, 2.69; 95% CI 1.67–4.34; grade III vs grade I, HR, 5.30; 95% CI 3.39–8.28; both P < 0.001), pre-/postoperative of chemotherapy (yes vs no, HR, 1.93; 95% CI 1.39–2.69; P < 0.001), tumor size > 10.4 cm (> 10.4 cm vs ≤ 10.4 cm, HR, 2.60; 95% CI 1.74–3.88; P < 0.001), no surgery operated on primary site (no vs yes, HR, 4.58; 95% CI 2.90–7.23; P < 0.001), single neoplasm (single vs multiple, HR, 4.419; 95% CI 2.67–7.31; P < 0.001) were independent risk factors for worse CSS. Median household income and local population size and degree of urbanization were excluded based on AIC in both multivariable Cox models.
3.3 Nomogram development and validation
Based on the develo** sets, nomogram models for OS and CSS were constructed respectively by including associated factors, according to the multivariate Cox regression model (Fig. 2). FNCLCC grade had the greatest significance and can contribute a maximum of 100 points in both OS and CSS nomograms.
The OS nomogram was internally and externally validated and the CSS nomogram was only internally validated due to incomplete external validation data. The C indices for OS nomogram (develo** set: 0.76 [95% CI 0.69–0.83]; internal validation set: 0.76 [95% CI 0.66–0.86]; external validation set: 0.69 [95% CI 0.58–0.80]; whole set: 0.75 [95% CI 0.70–0.80]) and CSS nomogram (develo** set: 0.81 [95% CI 0.74–0.87]; internal validation set: 0.80 [95% CI 0.70–0.90]; whole set: 0.80 [95% CI 0.75–0.85]) were higher than that of TNM staging system (OS: develo** set, 0.61 [95% CI 0.54–0.67]; internal validation set, 0.60 [95% CI 0.50–0.70]; whole set: 0.60 [95% CI 0.55–0.66]; CSS: develo** set, 0.60 [95% CI 0.53–0.67]; internal validation set, 0.55 [95% CI 0.45–0.65]; whole set: 0.58 [95% CI 0.53–0.64]), which demonstrated that the models had good discrimination ability.
Furthermore, we compared the predictive ability of the nomograms and TNM staging system through the AUC values of 3-, 5- and 7-year OS rates and CSS rates (Fig. 3). The AUC values of the nomogram for predicting 3-, 5- and 7-year OS were 0.84, 0.82, and 0.78 in the develo** set, 0.81, 0.82, and 0.83 in the internal validation set, and 0.68, 0.75, and 0.72 in the external validation set, respectively, while for the TNM staging system, the AUCs were 0.72, 0.66 and 0.65 in the develo** set, and 0.67, 0.67, and 0.64 in the internal validation set. The AUCs of the nomogram for predicting 3-, 5- and 7-year CSS were 0.88, 0.88, and 0.85 in the develo** set, and 0.86, 0.88, and 0.86 in the internal validation set, respectively, while for the TNM staging system, the AUCs were 0.63, 0.60 and 0.58 in the develo** set, and 0.57, 0.56, and 0.54 in the internal validation set.
ROC Curves Evaluated the Predictive Ability of the Nomograms (A–C, F, G) and TNM Staging System (D, E, H, I). A–C nomogram for predicting OS ROCs in develo** set, internal validation set, and external validation set respectively; D, E TNM staging system for predicting OS ROCs in develo** set and internal validation set; F, G nomogram for predicting CSS ROCs in develo** set and internal validation set; H, I TNM staging system for predicting CSS ROCs in develo** set and internal validation set
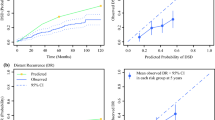
The calibration plots of the nomograms showed good consistency between the nomogram predicted and actual survival in the develo** sets and validation sets (Fig. 4). Using the median risk score 0.91 and 0.33 as the cutoff values for OS and CSS models, respectively, the patients from each cohort were stratified into high-risk and low-risk groups. The OS and CSS curves from each dataset for the different risk groups were significantly different (log-rank P < 0.001 in each dataset, Fig. 5). The same conclusion was obtained for the validation sets.
Calibration Plots for Estimating OS (A–I) and CSS (J–O) Survival Probability at 3, 5, and 7 Years. A–C Calibration plots in the OS develo** set; D–F Calibration plots in the OS internal validation set; G–I Calibration plots in the OS external validation set; J–L Calibration plots in the CSS develo** set; M–O Calibration plots in the CSS internal validation
Kaplan–Meier OS (A–C) and CSS (D–E) Curves of Different Risk Groups for RPS Patients Based on Risk Group Stratification in Each Cohort. A Kaplan–Meier OS Curves of develo** set; B Kaplan–Meier OS Curves of internal validation set; C Kaplan–Meier OS Curves of external validation set; D Kaplan–Meier CSS Curves of develo** set; E Kaplan–Meier CSS Curves of the internal validation set
4 Discussion
Most patients with RPS have grave prognoses with a high recurrence rate, yet their life expectancy remains greatly various [3, 19], which makes survival difficult to assess. As a rare tumor, there are many pathological types of RPS, the most common pathological types, such as liposarcoma and leiomyosarcoma, have been studied more frequently [20,21,22, This study developed and validated nomograms for predicting the prognosis of patients with RPS based on a large cohort, clinicopathological and epidemiological features were integrated to predict patients 3-, 5- and 7-year OS and CSS. The nomograms have been verified both internally and externally, and provide significantly better discrimination than the AJCC TNM staging system, hel** to guide monitoring and improve long-term survival outcomes, showing great potential for future clinical application. They can be used in patient consultations to provide accurate and useful information to both doctors and patients and accurately assess risk in individual patients and enhance clinical trial stratification. They also may be used in determining the proper timing for end-of-life discussions and/or hospice referrals.5 Conclusions
Data availability
The training datasets generated and analyzed during the current study are available in the SEER repository (https://seer.cancer.gov/); the validation datasets are available from the corresponding author on reasonable request.
References
Swallow CJ, Strauss DC, Bonvalot S, Rutkowski P, Desai A, Gladdy RA, Gonzalez R, Gyorki DE, Fairweather M, van Houdt WJ, Stoeckle E, Park JB, Albertsmeier M, Nessim C, Cardona K, Fiore M, Hayes A, Tzanis D, Skoczylas J, Ford SJ, Ng D, Mullinax JE, Snow H, Haas RL, Callegaro D, Smith MJ, Bouhadiba T, Stacchiotti S, Jones RL, DeLaney T, Roland CL, Raut CP, Gronchi A. Management of primary retroperitoneal sarcoma (RPS) in the adult: an updated consensus approach from the transatlantic australasian rps working group. Ann Surg Oncol. 2021;28:7873–88.
Álvarez Álvarez R, Manzano A, Agra Pujol C, Artigas Raventós V, Correa R, Cruz Jurado J, Fernandez JA, Garcia Del Muro X, Gonzalez JA, Hindi N, Lozano Lominchar P, Martínez-Trufero J, Méndez R, Muñoz M, Muñoz Casares C, Orbis Castellanos F, Orellana Fernandez R, Paniagua González M, Redondo A, Valverde Morales C, Asencio JM. Updated review and clinical recommendations for the diagnosis and treatment of patients with retroperitoneal sarcoma by the Spanish Sarcoma Research Group (GEIS). Cancers (Basel). 2023;15:3194.
Gamboa AC, Gronchi A, Cardona K. Soft-tissue sarcoma in adults: an update on the current state of histiotype-specific management in an era of personalized medicine. CA Cancer J Clin. 2020;70:200–29.
Bonvalot S, Gronchi A, Le Pechoux C, Swallow CJ, Strauss D, Meeus P, van Coevorden F, Stoldt S, Stoeckle E, Rutkowski P, Rastrelli M, Raut CP, Hompes D, De Paoli A, Sangalli C, Honore C, Chung P, Miah A, Blay JY, Fiore M, Stelmes JJ, Dei Tos AP, Baldini EH, Litiere S, Marreaud S, Gelderblom H, Haas RL. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21:1366–77.
Munoz P, Bretcha-Boix P, Artigas V, Asencio JM. Surgical principles of primary retroperitoneal sarcoma in the era of personalized treatment: a review of the frontline extended surgery. Cancers (Basel). 2022;14:4091.
von Mehren M, Kane JM, Bui MM, Choy E, Connelly M, Dry S, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Meyer C, Pappo AS, Parkes AM, Paz IB, Petersen IA, Poppe M, Riedel RF, Rubin B, Schuetze S, Shabason J, Sicklick JK, Spraker MB, Zimel M, Bergman MA, George GV. NCCN guidelines insights: soft tissue sarcoma, version 1.2021. J Natl Compr Canc Netw. 2020;18:1604–12.
Derbel O, Heudel PE, Cropet C, Meeus P, Vaz G, Biron P, Cassier P, Decouvelaere AV, Ranchere-Vince D, Collard O, De Laroche E, Thiesse P, Farsi F, Cellier D, Gilly FN, Blay JY, Ray-Coquard I. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive population-based cohort). PLoS ONE. 2017;12: e0158406.
Anaya DA, Lahat G, Wang X, **ao L, Pisters PW, Cormier JN, Hunt KK, Feig BW, Lev DC, Pollock RE. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann Oncol. 2010;21:397–402.
Gronchi A, Miceli R, Shurell E, Eilber FC, Eilber FR, Anaya DA, Kattan MW, Honore C, Lev DC, Colombo C, Bonvalot S, Mariani L, Pollock RE. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31:1649–55.
Raut CP, Callegaro D, Miceli R, Barretta F, Rutkowski P, Blay JY, Lahat G, Strauss DC, Gonzalez R, Ahuja N, Grignani G, Quagliuolo V, Stoeckle E, De Paoli A, Pillarisetty VG, Nessim C, Swallow CJ, Bagaria S, Canter R, Mullen J, Gelderblom HJ, Pennacchioli E, van Coevorden F, Cardona K, Fiore M, Fairweather M, Gronchi A. Predicting survival in patients undergoing resection for locally recurrent retroperitoneal sarcoma: a study and novel nomogram from TARPSWG. Clin Cancer Res. 2019;25:2664–71.
Callegaro D, Barretta F, Swallow CJ, Strauss DC, Bonvalot S, Honore C, Stoeckle E, van Coevorden F, Haas R, Rutkowski P, Schrage Y, Fairweather M, Conti L, Vassos N, Gladdy RA, Ng D, van Houdt WJ, Miceli R, Raut CP, Gronchi A. Longitudinal prognostication in retroperitoneal sarcoma survivors: development and external validation of two dynamic nomograms. Eur J Cancer. 2021;157:291–300.
Ardoino I, Miceli R, Berselli M, Mariani L, Biganzoli E, Fiore M, Collini P, Stacchiotti S, Casali PG, Gronchi A. Histology-specific nomogram for primary retroperitoneal soft tissue sarcoma. Cancer. 2010;116:2429–36.
Tan MC, Brennan MF, Kuk D, Agaram NP, Antonescu CR, Qin LX, Moraco N, Crago AM, Singer S. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. 2016;263:593–600.
Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: surveillance, epidemiology, and end results (SEER) database. JAMA Surg. 2018;153:588–9.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9.
Akaike H. Information theory and an extension of the maximum likelihood principle. In: Parzen E, Tanabe K, Kitagawa G, editors. Selected papers of Hirotugu Akaike. New York: Springer; 1998. p. 199–213.
Venables WN, Ripley BD, Venables WN. Modern applied statistics with S. 4th ed. New York: Springer; 2002.
Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000.
Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, Van Coevorden F, Rutkowski P, Callegaro D, Hayes AJ, Honore C, Fairweather M, Cannell A, Jakob J, Haas RL, Szacht M, Fiore M, Casali PG, Pollock RE, Raut CP. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS Working Group. Ann Surg. 2016;263:1002–9.
Tyler R, Wanigasooriya K, Taniere P, Almond M, Ford S, Desai A, Beggs A. A review of retroperitoneal liposarcoma genomics. Cancer Treat Rev. 2020;86: 102013.
Zuco V, Pasquali S, Tortoreto M, Brich S, Percio S, Dagrada GP, Colombo C, Sanfilippo R, Lauricella C, Gounder M, El Bezawy R, Barisella M, Dei Tos AP, Casali PG, Gronchi A, Stacchiotti S, Zaffaroni N. Selinexor versus doxorubicin in dedifferentiated liposarcoma PDXs: evidence of greater activity and apoptotic response dependent on p53 nuclear accumulation and survivin down-regulation. J Exp Clin Cancer Res. 2021;40:83.
Almond LM, Tirotta F, Tattersall H, Hodson J, Cascella T, Barisella M, Marchiano A, Greco G, Desai A, Ford SJ, Gronchi A, Fiore M, Morosi C. Diagnostic accuracy of percutaneous biopsy in retroperitoneal sarcoma. Br J Surg. 2019;106:395–403.
Chen H, Wei J, Wang R, **ang Y, Fan C. Retroperitoneal Liposarcoma on 99mTc-DTPA Renal Scintigraphy. Clin Nucl Med. 2020;45:129–30.
Tyler R, Dilworth MP, James J, Blakeway D, Stockton JD, Morton DG, Taniere P, Gourevitch D, Desai A, Beggs AD. The molecular landscape of well differentiated retroperitoneal liposarcoma. J Pathol. 2021;255:132–40.
Xu C, Yan L, Guan X, Wang Z, Wu J, Lv A, Liu D, Liu F, Dong B, Zhao M, Jia L, Tian X, Hao C. Tsp2 facilitates tumor-associated fibroblasts formation and promotes tumor progression in retroperitoneal liposarcoma. Int J Biol Sci. 2022;18:5038–55.
Tucci JJ, Dashti NK, Cates JMM. A proposed staging system for improved prognostication of MDM2-amplified liposarcoma. Am J Surg Pathol. 2021;45:101–7.
Marko J, Wolfman DJ. Retroperitoneal leiomyosarcoma from the radiologic pathology archives. Radiographics. 2018;38:1403–20.
Carvalho JC, Thomas DG, Lucas DR. Cluster analysis of immunohistochemical markers in leiomyosarcoma delineates specific anatomic and gender subgroups. Cancer. 2009;115:4186–95.
Kezios KL, Lu P, Calonico S, Al Hazzouri AZ. History of low hourly wage and all-cause mortality among middle-aged workers. JAMA. 2023;329:561–73.
Landon BE, Hatfield LA, Bakx P, Banerjee A, Chen YC, Fu C, Gordon M, Heine R, Huang N, Ko DT, Lix LM, Novack V, Pasea L, Qiu F, Stukel TA, Uyl-de Groot C, Yan L, Weinreb G, Cram P. Differences in treatment patterns and outcomes of acute myocardial infarction for low- and high-income patients in 6 countries. JAMA. 2023;329:1088–97.
Gundle KR, Kafchinski L, Gupta S, Griffin AM, Dickson BC, Chung PW, Catton CN, O’Sullivan B, Wunder JS, Ferguson PC. Analysis of margin classification systems for assessing the risk of local recurrence after soft tissue sarcoma resection. J Clin Oncol. 2018;36:704–9.
von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Pollack SM, Poppe M, Riedel RF, Schuetze S, Shabason J, Sicklick JK, Spraker MB, Zimel M, Hang LE, Sundar H, Bergman MA. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:815–33.
Ecker BL, Peters MG, McMillan MT, Sinnamon AJ, Zhang PJ, Fraker DL, Levin WP, Roses RE, Karakousis GC. Preoperative radiotherapy in the management of retroperitoneal liposarcoma. Br J Surg. 2016;103:1839–46.
Callegaro D, Raut CP, Ajayi T, Strauss D, Bonvalot S, Ng D, Stoeckle E, Fairweather M, Rutkowski P, van Houdt WJ, Gelderblom H, Sangalli C, Hayes A, Honore C, Gladdy RA, Fau M, Haas R, Tzanis D, Miah AB, Chung P, Baldini EH, Marreaud S, Litiere S, Swallow CJ, Gronchi A. Preoperative radiotherapy in patients with primary retroperitoneal sarcoma: EORTC-62092 trial (STRASS) versus off-trial (STREXIT) results. Ann Surg. 2022;278(1):127–34.
Datta J, Ecker BL, Neuwirth MG, Geha RC, Fraker DL, Roses RE, Karakousis GC. Contemporary reappraisal of the efficacy of adjuvant chemotherapy in resected retroperitoneal sarcoma: evidence from a nationwide clinical oncology database and review of the literature. Surg Oncol. 2017;26:117–24.
Kelly CM, Antonescu CR, Bowler T, Munhoz R, Chi P, Dickson MA, Gounder MM, Keohan ML, Movva S, Dholakia R, Ahmad H, Biniakewitz M, Condy M, Phelan H, Callahan M, Wong P, Singer S, Ariyan C, Bartlett EK, Crago A, Yoon S, Hwang S, Erinjeri JP, Qin LX, Tap WD, D’Angelo SP. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: a phase 2 clinical trial. JAMA Oncol. 2020;6:402–8.
Gronchi A, Palmerini E, Quagliuolo V, Martin Broto J, Lopez Pousa A, Grignani G, Brunello A, Blay JY, Tendero O, Diaz Beveridge R, Ferraresi V, Lugowska I, Merlo DF, Fontana V, Marchesi E, Braglia L, Donati DM, Palassini E, Bianchi G, Marrari A, Morosi C, Stacchiotti S, Bague S, Coindre JM, Dei Tos AP, Picci P, Bruzzi P, Casali PG. Neoadjuvant chemotherapy in high-risk soft tissue sarcomas: final results of a randomized trial from Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J Clin Oncol. 2020;38:2178–86.
C.D.M. Fletcher, World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press; 2013.
Gronchi A, Miceli R, Colombo C, Stacchiotti S, Collini P, Mariani L, Sangalli C, Radaelli S, Sanfilippo R, Fiore M, Casali PG. Frontline extended surgery is associated with improved survival in retroperitoneal low- to intermediate-grade soft tissue sarcomas. Ann Oncol. 2012;23:1067–73.
Bonvalot S, Miceli R, Berselli M, Causeret S, Colombo C, Mariani L, Bouzaiene H, Le Pechoux C, Casali PG, Le Cesne A, Fiore M, Gronchi A. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010;17:1507–14.
Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY, Laplanche A. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–7.
Gronchi A, Lo Vullo S, Fiore M, Mussi C, Stacchiotti S, Collini P, Lozza L, Pennacchioli E, Mariani L, Casali PG. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30.
Lochan R, French JJ, Manas DM. Surgery for retroperitoneal soft tissue sarcomas: aggressive re-resection of recurrent disease is possible. Ann R Coll Surg Engl. 2011;93:39–43.
R.P.S.W.G. Trans-Atlantic. Management of primary retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol. 2015;22:256–63.
Ma SJ, Oladeru OT, Farrugia MK, Shekher R, Iovoli AJ, Singh AK. Evaluation of preoperative chemotherapy or radiation and overall survival in patients with nonmetastatic, resectable retroperitoneal sarcoma. JAMA Netw Open. 2020;3: e2025529.
Italiano A, Penel N, Robin YM, Bui B, Le Cesne A, Piperno-Neumann S, Tubiana-Hulin M, Bompas E, Chevreau C, Isambert N, Leyvraz S, du Chatelard PP, Thyss A, Coindre JM, Blay JY. Neo/adjuvant chemotherapy does not improve outcome in resected primary synovial sarcoma: a study of the French Sarcoma Group. Ann Oncol. 2009;20:425–30.
Bonvalot S, Raut CP, Pollock RE, Rutkowski P, Strauss DC, Hayes AJ, Van Coevorden F, Fiore M, Stoeckle E, Hohenberger P, Gronchi A. Technical considerations in surgery for retroperitoneal sarcomas: position paper from E-Surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann Surg Oncol. 2012;19:2981–91.
Haas RLM, Bonvalot S, Miceli R, Strauss DC, Swallow CJ, Hohenberger P, van Coevorden F, Rutkowski P, Callegaro D, Hayes AJ, Honore C, Fairweather M, Gladdy R, Jakob J, Szacht M, Fiore M, Chung PW, van Houdt WJ, Raut CP, Gronchi A. Radiotherapy for retroperitoneal liposarcoma: a report from the Transatlantic Retroperitoneal Sarcoma Working Group. Cancer. 2019;125:1290–300.
Bremjit PJ, Jones RL, Chai X, Kane G, Rodler ET, Loggers ET, Pollack SM, Pillarisetty VG, Mann GN. A contemporary large single-institution evaluation of resected retroperitoneal sarcoma. Ann Surg Oncol. 2014;21:2150–8.
R.P.S.W.G. Trans-Atlanctic. Management of recurrent retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol. 2016;23:3531–40.
Tseng WW, Madewell JE, Wei W, Somaiah N, Lazar AJ, Ghadimi MP, Hoffman A, Pisters PW, Lev DC, Pollock RE. Locoregional disease patterns in well-differentiated and dedifferentiated retroperitoneal liposarcoma: implications for the extent of resection? Ann Surg Oncol. 2014;21:2136–43.
Funding
This research was funded by National Natural Science Foundation of China, Grant Number 8227102083 and Joint Laboratory of **amen University School of Medicine-Shanghai Jiangxia Blood Technology Co., Ltd, Grant Number XDHT2020010C.
Author information
Authors and Affiliations
Contributions
Conceptualization, JZ, AZ and XX; methodology, JZ, AZ, XX and FM; data curation, AZ, YM and JZ, validation, JZ, XX and ZW; investigation, JZ, AZ, ZL and XK; writing—original draft preparation, all authors; writing—review and editing, all authors; visualization, JZ, XK and YR; supervision, WQL, WGL, and JZ; funding acquisition, WGL. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Decla-ration of Helsinki, and approved by the Institutional Review Board of South Hospital of the Zhongshan Hospital/Shanghai Public Health Clinical Center, Fudan University, Shanghai, China. The research was carried out following the guidelines of the ethics committee listed in the ethics statement. Patient information is completely anonymous and insufficient to identify the patient’s identity. Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Results of the Multivariable Cox Models incorporating income for OS and CSS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, J., Zhuang, A., **a, X. et al. Nomogram development and external validation for predicting overall survival and cancer-specific survival in patients with primary retroperitoneal sarcoma: a retrospective cohort study. Discov Onc 14, 197 (2023). https://doi.org/10.1007/s12672-023-00804-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00804-1