Abstract
This study aimed to explore naringin’s potential to promote the osteogenic differentiation of MC3T3-E1 under oxidative stress. It delved into Nar’s connection with the Wnt/β-catenin and PI3K/Akt signaling pathways. Initially, 2911 OP-related genes were analyzed, revealing close ties with the PI3K/Akt and Wnt pathways alongside oxidative stress. Nar’s potential targets—ESR1, HSP90AA1, and ESR2—were identified through various databases and molecular docking studies confirmed Nar’s affinity with ESR1 and HSP90AA1. Experiments established optimal concentrations for Nar and H2O2. H2O2 at 0.3 mmol/L damaged MC3T3-E1 cells, alleviated by 0.1 µmol/L Nar. Successful establishment of oxidative stress models was confirmed by DCFH-DA probe and NO detection. Nar exhibited the ability to enhance osteogenic differentiation, counteracting oxidative damage. It notably increased osteoblast-related protein expression in MC3T3-E1 cells under oxidative stress. The study found Nar’s positive influence on GSK-3β phosphorylation, β-catenin accumulation, and pathway-related protein expression, all critical in promoting osteogenic differentiation. The research concluded that Nar effectively promotes osteogenic differentiation in MC3T3-E1 cells under oxidative stress. It achieved this by activating the Wnt/β-catenin and PI3K/Akt pathways, facilitating GSK-3β phosphorylation, and enhancing β-catenin accumulation, pivotal in osteogenesis.
Similar content being viewed by others
Introduction
The skeletal system plays a crucial role through structural support, organ protection, and facilitating movement in the body. Osteoblast-mediated bone formation and osteoclast-mediated bone resorption are key homeostatic processes that occur in adult bones to replace aging tissues and repair damage1. Owing to the rapid aging of populations worldwide, the prevalence of OP continues to rise. Therefore, the prevention and treatment of OP have become essential issues requiring urgent attention in modern medicine.
OP is mainly caused by abnormal bone resorption rather than formation2, whereby increases in bone resorption lead to the acceleration of bone loss. Therefore, inhibiting bone resorption or activating bone formation is an effective way to treat osteoporosis. In recent years, traditional Chinese medicines' prophylactic and therapeutic effects on OP have attracted widespread attention from researchers, especially since the active ingredients typically have few side effects and high cost and time efficiency3.
The accumulation of free radicals, such as or reactive oxygen species (ROS), is a major cause of OP-related morbidity4,5. ROS, as an important intermediate product of oxidative stress, are related to osteogenic differentiation and bone regeneration6. Low ROS levels play a role in regulating signaling mediators conducive for bone regeneration. In contrast, excess ROS production often suppresses Wnt/β-catenin signaling and activates FoxO signaling, resulting in impaired bone regeneration21. Oxidative stress is an important mechanism affecting OP pathogenesis that can cause excessive bone loss in the human body by altering osteoblast, osteoclast, and bone marrow stromal cell (BMSC) proliferation and metabolism, resulting in an imbalance in bone homeostasis22. Currently, using anti-oxidants to inhibit oxidative stress and delay the development of OP is an important strategy for OP treatment. However, alternative anti-oxidant therapies with improved clinical efficacy and cost-efficiency with fewer side effects are needed to improve patients' quality of life with OP.
This study investigated the specific mechanism of OP pathogenesis by using the GEO2R and R language packages to analyze the GEO dataset comprehensively. A total of 2911 OP-related DEGs then underwent enrichment analyses using KEGG and GO. KEGG analysis revealed that OP-related DEGs were mainly enriched in the PI3K/Akt and Wnt signaling pathways, which was consistent with numerous studies reporting a role for the PI3K/Akt and Wnt signaling in osteogenic differentiation23,24. GO function analysis showed that OP-related DEGs were mainly enriched in response to oxidative stress, ROS, and H2O2 in BP and were mainly enriched in binding growth factor receptors, peroxisome proliferator-activated receptors, and NO synthase in MF. NO is a typical free radical with strong reactivity that can react with superoxide anions to form nitroso complexes, causing high oxidative stress25. This coincides with the BP and MF results, which confirmed that oxidative stress caused by excessive ROS production was a major cause of OP and suggested that eliminating ROS may be the key to OP treatment26. In addition, the CC results showed that OP-related DEGs were closely related to intercellular junctions and the nuclear envelope.
Among the 29 potential Nar targets found in this study, the following 10 core targets were selected based on degree value: ER1, HSP90AA1, ESR2, ABCG2, DNMT1, CA12, PTPN1, CA9, TUBA1A, and CYP1A2. Upon determining the overlap between OP-related proteins and these core targets, we found 3 OP-related Nar potential targets: ESR1, HSP90AA1, and ESR2. In particular, our findings implicated ESR1 as a key target for Nar's action. Previous studies have discussed the potential targets of Nar and addressed molecular docking between target proteins and drug molecules27; however, we further refined these Nar targets and revealed their important roles in the present study. Specifically, molecular docking and protein–ligand interaction analyses revealed that Nar had a good affinity for ESR1 and HSP90AA1 and identified multiple protein–ligand interactions.
ESR activation has been proposed as a positive regulator of the Wnt/β-catenin and PI3K/Akt signaling, with a cross-talk relationship between both pathways reported28,29. Since the primary OP is characterized by steroid hormone deficiency and high oxidative stress, and based on the network pharmacology results of this study, we speculated that Nar may regulate Wnt/β-catenin and PI3K/Akt signaling by activating ESR and subsequently reverse oxidative stress-impaired osteogenic differentiation.
This study used bioinformatics to confirm that OP is closely related to oxidative stress and the PI3K/Akt and Wnt signaling pathways. Nar, one of the main components of traditional Chinese medicine, has anti-inflammatory, anti-oxidant, bone-promoting, and other pharmacological properties. The first study to report Nar-mediated promotion of osteogenic differentiation used BMSCs cultured with varying concentrations of Nar30. In our previous study, Nar promoted the osteogenic differentiation of human adipose mesenchymal stem cells via the Wnt/β-catenin signaling pathway under oxidative damage conditions, further highlighting its anti-oxidant and osteogenic effects8. The results showed that the maturation process of MC3T3-E1 cells could be divided into three stages: cell proliferation, early differentiation (characterized by ALP secretion), and mineralized nodule formation. As these three stages were similar to the differentiation and maturation of normal osteoblasts and were the experimental models for the study of osteoblast maturation31, MC3T3-E1 cells were used in the present study to explore further the relationship between Nar-mediated osteogenic differentiation and oxidative stress resistance and its related mechanisms.
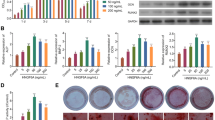
Osteoblasts require moderate physical activity for bone formation and remodeling. An increase in osteoblast quantity can be achieved by promoting the proliferation and differentiation of osteoblast progeny cells and reducing the death of mature osteoblasts32. The results of our CCK-8 assay showed that 0.1 μmol/L Nar could effectively improve the proliferative activity of MC3T3-E1 cells. However, the viability of MC3T3-E1 cells decreased significantly after treatment with 0.3 mmol/L H2O2, with a cell survival rate of 75%.
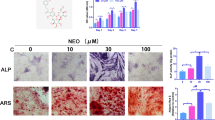
As an early marker of osteoblast differentiation, ALP promotes physiological calcium salt deposition by hydrolyzing glycerophosphate sodium, which plays an important role in mineralization during osteogenesis33. In the current study, osteogenesis was induced in MC3T3-E1 cells, and ALP activity, expression of intracellular osteoblast-related proteins, and extracellular calcium salt deposition were measured. The results showed that the osteogenic ability of MC3T3E1 cells decreased under oxidative stress, while Nar could improve the osteogenic differentiation inhibited by oxidative stress to a certain extent.
Wnt/β-catenin signaling pathway is a classical pathway that determines cell proliferation and differentiation and influences cell function by regulating β-catenin levels and subcellular localization34. Without the 6Wnt ligand, β-catenin is phosphorylated at its NH2 terminal and degraded by a multiprotein complex comprising GSK-3β, APC, and Axin's skeleton protein35,36. Activation of the Wnt pathway prevents the formation of this multiprotein complex and subsequent β-catenin degradation36. Instead, it accumulates and translocates to the nucleus to activate the transcription of a series of genes. This study found that under oxidative stress, Nar increased the expression of the osteogenic proteins Runx2 and OPN, and Wnt/β-catenin signalling-related proteins, while Wnt-C59 inhibited this effect. These results suggest that Nar promotes the osteogenic differentiation of MC3T3-E1 cells inhibited by oxidative stress, potentially via the Wnt/β-catenin signaling pathway.
The PI3K signal transduction process is an important pathway in regulating cell proliferation, differentiation, and apoptosis37. In a previous study, H2O2 was used to establish a model of oxidative stress in BMSCs and explored the effects of ebselen treatment. The results showed that ebselen-promoted osteogenic differentiation was inhibited by oxidative stress via the PI3K/Akt signaling pathway38. However, it has not yet been reported whether Nar, with its strong anti-oxidant properties, can similarly promote oxidative stress-impaired osteogenic differentiation. In the current study, Nar increased the expression of PI3K/Akt signaling proteins related to osteogenic differentiation in MC3T3-E1 cells with oxidative stress injury, and this effect was blocked by the PI3K/Akt signaling inhibitor, LY294002. Therefore, Nar may promote osteogenic differentiation inhibited by oxidative stress via the PI3K/Akt signaling pathway.
This study had several limitations. First, our hypothesis was only tested in vitro and needs to be further verified in vivo. Second, whether and how Wnt/β-catenin and PI3K/Akt signaling interact remains unclear and warrants further exploration.
In conclusion, this study explored how Nar promotes osteogenic differentiation by establishing an oxidative stress model using H2O2 and evaluating Wnt/β-catenin and PI3K/Akt signalling-related proteins in OP samples. Notably, Nar could induce the phosphorylation of GSK-3β by activating Wnt/β-catenin and PI3K/Akt signaling, leading to the accumulation of β-catenin, and ultimately activating the expression of osteogenic markers. These results suggest that Nar promotes the osteogenic differentiation of MC3T3-E1 cells inhibited by oxidative stress by activating Wnt/β-catenin and PI3K/Akt signaling pathways and presents Nar as a potential treatment for OP. Owing to the development of traditional Chinese medicine and its increasing use in modern medicine, we expect breakthroughs that will establish an effective clinical treatment strategy for OP management and improve patients' quality of life soon.
Materials and methods
Data collection and processing
Using the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/GEO/) with the keyword ‘osteoporosis’ for retrieval, we downloaded the GSE3595839 dataset for OP and non-OP samples. Using a standardized GEO2R gene microarray (http://www.ncbi.nlm.nih.Gov/geo/geo2r), we compared the DEGs between both samples and defined those that had a |logFC (fold change)|> 1 and false discovery rate − log10(P value) < 1.3 (FDR < 0.05) as OP-related DEGs. The data were visualized using R 4.3.2’s ggplot2 3.5.0 and pheatmap 1.0.12 packages (R Core Team, Austria).
Enrichment analysis of DEGs
The General Database for Annotation, Visualisation, and Integrated Discovery40 (DAVID; https://david.ncifcrf.gov) was used for Gene Ontology (GO) and Kyoto Encyclopaedia of Genes and Genomes41 (KEGG) functional enrichment analysis. The GO analysis consisted of three components: biological process (BP), molecular function (MF), and cellular component (CC). The data were visualised using the ggplot2 3.5.0 package. P-values < 0.05 were considered statistically significant.
Screening of Nar core targets
“Naringin” was used as a keyword in PubChem (https://pubchem.ncbi.nlm.nih.gov/) to retrieve the Simplified Molecular Input Line Entry System (SMILES) chemical formula. Then, using this formula, we screened the TargetNet database (http://targetnet.scbdd.com/home/index/) and only included models with an area under the curve (AUC) cut-off value of 0.7. The potential targets of Nar were identified using extended-connectivity (ECFP4) fingerprint retrieval, and a protein–protein interaction (PPI) network was drawn using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database. The degree value of each node in the PPI network was sorted using Cytoscape_v3.10.0-SNAPSHOT42 (Cytoscape Team, USA). The top 10 targets with the highest scores were selected and defined as the core targets.
Screening of the potential OP-associated drug targets
OP-related proteins were searched in Comparative Toxicogenomics Database (CTD), DisGeNET, and GeneCards using “osteoporosis” as the keyword. The core target intersection was determined using an online Venn diagram tool (http://bioinformatics.psb.ugent.be/webtools/Venn/), and the potential Nar action targets associated with OP were obtained.
Molecular docking
Oestrogen receptor 1 (ESR1) and heat shock protein 90 alpha family class A member 1 (HSP90AA1) proteins obtained from the Protein Data Bank (PDB) (https://www.rcsb.org/) were pre-treated for structural optimization using molecular operating environment software (MOE; Chemical Computing Group, Canada) and the sitefinder function was used to identify the binding pockets For molecular docking, the receptor was set as ESR1 or HSP90AA1, site as site 1, and the ligand as Nar. The results of the analysis were visualized using PyMOL 2.6.0 software43 (Schrödinger, Inc., USA).
Cell culture
Resuscitated MC3T3-E1 cells (EK-Bioscience, China) were cultured in minimum essential medium alpha (α-MEM; Shanghai BasalMedia Technologies Co., Ltd., China) medium containing 10% fetal bovine serum (Nan**g SenBeiJia Biological Technology Co., Ltd., China) and 1% penicillin–streptomycin solution (Nan**g SenBeiJia Biological Technology Co., Ltd., China) at 37℃ in a 5% CO2 incubator (Sanyo, Japan). After the cells reached 80%–90% confluency, they were treated with 0.25% trypsin/0.02% ethylenediaminetetraacetic acid solution (Sigma, USA) and re-plated at a dilution of 1:3.
CCK-8 assay
MC3T3-E1 cells in the logarithmic growth phase were seeded in 96-well plates with 1 × 104 cells/well and cultured for 24 h. Cells were treated with a low-serum medium containing various Nar concentrations (Sigma, USA) or H2O2 (Sigma, USA) for 24 h or with Nar following 24 h of H2O2 treatment for 24, 48, and 72 h. Cell counting kit-8 (CCK-8) reagent (Meilun Biological, China) was added to the cells and incubated for 2 h. Absorbances were measured at 450 nm using a microplate reader (Molecular Devices, USA). Cell survival rate was calculated using the optical density (OD) values as follows:
Cytotoxicity assay
MC3T3-E1 cells were subjected to 0.3 mM H2O2 and 0.1 µM Nar for 24 h to detect lactate dehydrogenase (LDH) (Elabscience, China) production. The MC3T3-E1 cells were seeded in 96-well plates and cultured for 24 h. Cells were treated according to the following groups: control, induction, H2O2, Nar, and H2O2 + Nar. Then, 10 μL of lysate was added to the control well with maximum enzyme activity, and the cells were cultured for another 1 h after blowing and mixing. After centrifugation at 400 × g for 5 min, 50 μL of supernatant to be measured and 50 μL of reaction working solution was added to each well, and the microplate was shaken. After incubating at 37 °C for 10 min, the reaction termination solution was added, and the absorbance was measured at 450 nm using a microplate reader. Cytotoxicity was calculated as follows:
ROS assay
After cleaning with phosphate-buffered saline (PBS), a 2'-7'dichlorofluorescin diacetate (DCFH-DA) (Applygen Technologies, China) probe diluted with α-MEM (final concentration of 10 μM) was added to the MC3T3-E1 cells in 6-well plates and incubated at 37 °C for 30 min in the dark. During this period, the probe was gently shaken every 5 min to ensure full contact with the cells. The cells were washed three times and then re-suspended with α-MEM. ROS levels were recorded under a fluorescence microscope within 30 min at 525 nm to determine the establishment of the oxidative stress model. Fluorescence staining used ImageJ 13.0.6 software44 to detect fluorescence brightness as ROS expression. The cell suspension was detected by fluorescent enzyme, the absorbance was measured at 520 nm, and the results were expressed by fluorescence value.
Nitric oxide assay
Dilute the standard in the kit to 800, 400, 200, 100, 50, 25, 12.5, 6.25, 3.13, 1.57 μM, the OD values of each group were detected by microplate reader, and the concentration calculation formula was obtained by making a standard curve. In 96-well plate, 50 μL control(10%FBS-α-MEM) and 50 μL Griess reagent were added and incubated at room temperature in the dark for 5 min. Then, the absorbance was detected using at 540 nm. A standard curve was drawn, the OD values of the sample group were substituted into the formula to calculate the NO expression in the group, and the concentration of NO was calculated to confirm the establishment of the oxidative stress model.
Alkaline phosphatase staining
Osteogenesis was induced in the MC3T3-E1 cells seeded in 6-well plates according to the different groups. Cell culture was performed with α-MEM in the control group, with basic osteogenic differentiation medium (α-MEM, 10% FBS, 50 μg/mL ascorbic acid, and 10 mM sodium β-glycerophosphate) in the induction group, and in the hydrogen peroxide group, basic osteogenic differentiation medium was added after 24 h of H2O2 action. For the Nar group, we added Nar to the basal osteogenic differentiation medium, while for the H2O2 + Nar group, we added the above Nar-containing osteogenic differentiation medium after 24 h of H2O2 action. and stained using the BCIP/NBT alkaline phosphatase chromogenic kit (Beyotime, China). After 2 weeks, the medium was removed, and the cells were washed three times with PBS for 3–5 min each, fixed with 4% paraformaldehyde for 15 min, and washed a further three times with PBS. Then, 1 mL of the staining working solution was added to each well and incubated at room temperature for 20 min in the dark. After removing the working solution, the staining solution was washed three times with double distilled H2O and imaged under an inverted microscope.
Alizarin red S staining
Osteogenesis was induced in the MC3T3-E1 cells seeded in 6-well plates according to different groups and then stained with the osteoblast mineralized nodule staining kit (Beyotime, China). After 21 days, the medium was discarded, and the cell surface was lightly washed three times with PBS for 3–5 min each, fixed with 4% paraformaldehyde for 15 min, and washed a further three times with double distilled H2O. Alizarin Red S staining working solution (1 mL) was added to each well and the cells were stained for 30 min. After discarding the solution, the cells were washed three times with ddH2O and imaged under an inverted microscope.
Alkaline phosphatase activity assay
Osteogenesis was induced in MC3T3-E1 cells in 6-well plates for 5, 7, and 9 days, according to different groups. The cells were harvested and lysed, and the remaining supernatant was collected. The alkaline phosphatase (ALP) detection kit (Beyotime, China) was used following the manufacturer’s instructions. Absorbances were measured at 405 nm, and the activity of ALP was calculated (Table S1).
Western blot
Osteogenesis was induced in the MC3T3-E1 cells for 14 days, and they were treated according to different groups. Total protein was extracted using radioimmunoprecipitation assay lysis buffer (Beyotime, China), quantified using the bicinchoninic acid method (Applygen Technologies Inc., China), and denatured by boiling after adding loading buffer. Whole-cell protein extracts were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and analyzed using Western blot (WB). The amount of protein was loaded with 10 μg/well. The PVDF membranes (0.22 μm) were then blocked with 5% BSA for 2 h. The following primary antibodies were prepared at 1:1000 after washing the membrane and incubated overnight at 4 °C: anti-GAPDH (Affinity, USA), anti-PI3K (Affinity, China), anti-p-PI3K (Affinity, China), anti-Akt (Affinity, China), anti-p-Akt (Affinity, China), anti-GSK-3β (Affinity, China), anti-p-GSK-3β (Affinity, China), anti-LRP-5 (Abcam, China), anti-β-catenin (Cell Signalling Technology, USA), anti-Runx2 (Affinity, China), and anti-OPN (Beyotime, China). The membranes were then washed three times with PBS-Tween, incubated with a secondary antibody for 2 h at room temperature, and washed three more times. Enhanced chemiluminescence solution (Applygen Technologies, China) was then applied, and the relative image intensity was calculated using ImageJ software (Table S1).
Statistical analysis
Data are expressed as the mean ± standard deviation. All statistical analyses were performed using SPSS 25.0 (IBM Corp., USA) and GraphPad Prism 8.0 (GraphPad Software, Inc., USA). The independent sample t-test was used for comparison between two groups. A one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was used to compare multiple groups. P-values < 0.05 were considered statistically significant.
Ethics approval and consent to participate
The data for the bioinformatics analyses in this study were obtained from online databases, and the cell lines were purchased by the company, so no ethical approvals were involved.
Data availability
The data during the current study are available from the corresponding author, H.W., upon reasonable request.
References
Hasegawa, T. et al. Identification of a novel arthritis-associated osteoclast precursor macrophage regulated by FoxM1. Nat. Immunol. 20(12), 1631–1643 (2019).
Sleeman, A. & Clements, J. N. Abaloparatide: A new pharmacological option for osteoporosis. Am. J. Health Syst. Pharm. 76(3), 130–135 (2019).
**, C. et al. Corynoline suppresses osteoclastogenesis and attenuates ROS activities by regulating NF-κB/MAPKs and Nrf2 signaling pathways. J. Agric. Food Chem. 72, 8149–8166 (2024).
Moya-Angeler, J., Lane, J. M. & Rodriguez, J. A. Metabolic bone diseases and total hip arthroplasty: Preventing complications. J. Am. Acad. Orthop. Surg. 25(11), 725–735 (2017).
Marques-Carvalho, A. et al. The role of reactive oxygen species in bone cell physiology and pathophysiology. Bone Rep. 19, 101664 (2024).
Geng, Q. et al. Pyrroloquinoline quinone prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Int. J. Biol. Sci. 15(1), 58–68 (2019).
**ong, Yi. et al. FOXO1 differentially regulates bone formation in young and aged mice. Cell Sign. 99, 110438 (2022).
Wang, L. et al. Naringin protects human adipose-derived mesenchymal stem cells against hydrogen peroxide-induced inhibition of osteogenic differentiation. Chem. Biol. Interact. 242, 255–261 (2015).
Shirani, K. et al. Protective effects of naringin against drugs and chemical toxins induced hepatotoxicity: A review. Phytother. Res. 34(8), 1734–1744 (2020).
Ni, K. et al. Naringin as a plant-derived bitter tastant promotes proliferation of cultured human airway epithelial cells via activation of TAS2R signaling. Phytomedicine 84, 153491 (2021).
Wang, F. et al. Naringin alleviates atherosclerosis in ApoE Mice by regulating cholesterol metabolism involved in gut microbiota remodeling. J. Agric. Food Chem. 68(45), 12651–12660 (2020).
Zhu, L. et al. Effects of Naringenin on inflammation in complete freund's adjuvant-induced arthritis by regulating Bax/Bcl-2 balance. Inflammation 38(1), 245–251 (2015).
Chen, Y. et al. Mucoactive effects of naringin in lipopolysaccharide-induced acute lung injury mice and beagle dogs. Environ Toxicol Pharmacol. 38(1), 279–287 (2014).
Zhang, J. et al. Naringin ameliorates diabetic nephropathy by inhibiting NADPH oxidase 4. Eur. J. Pharmacol. 804, 1–6 (2017).
Li, X. L. et al. A naringin- and icariin-contained herbal formula, gushukang, ameliorated aged osteoporosis of aged mice with high calcium intake. Am. J. Chin. Med. 48(7), 1671–1691 (2020).
Li-**, N. et al. Naringin alleviates H2O2-induced apoptosis via the PI3K/Akt pathway in rat nucleus pulposus-derived mesenchymal stem cells. Connect. Tissue Res. 61(6), 554–567 (2020).
Xu, Q., Zhang, Z. F. & Sun, W. X. Effect of naringin on monosodium iodoacetate-induced osteoarthritis pain in rats. Med. Sci. Monit. 23, 3746–3751 (2017).
Ortiz-Andrade, R. R. et al. Anti-diabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Obes. Metab. 10(11), 1097–1104 (2008).
Guo, M. et al. Naringin promotes osteogenic/odontogenic differentiation of dental pulp stem cells via Wnt/β-catenin. Evid. Based Complement. Alternat. Med. 2022, 4505471 (2022).
Qiu, Z. C. et al. 8-prenylgenistein exerts osteogenic effects via ER α and Wnt-dependent signaling pathway. Exp. Cell Res. 395(1), 112186 (2020).
Słupski, W., Jawień, P. & Nowak, B. Botanicals in postmenopausal osteoporosis. Nutrients 13(5), 1609 (2021).
Yang, Y. et al. Oxidative stress induces downregulation of TP53INP2 and suppresses osteogenic differentiation of BMSCs during osteoporosis through the autophagy degradation pathway. Free Radic. Biol. Med. 166, 226–237 (2021).
Gan, J. et al. The Development of Naringin for Use against Bone and Cartilage Disorders. Molecules. 28(9), 3716 (2023).
Wu, J. B. et al. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur J Pharmacol. 588(2-3), 333–341 (2008).
Kaur, S. et al. Methods to detect nitric oxide and reactive nitrogen species in biological sample. Methods Mol. Biol. 2413, 69–76 (2022).
Kimball, J. S., Johnson, J. P. & Carlson, D. A. Oxidative stress and osteoporosis. J. Bone Jt. Surg. Am. 103(15), 1451–1461 (2021).
Yu, X. et al. Network pharmacology integrated with molecular docking explores the mechanisms of naringin against osteoporotic fracture by regulating oxidative stress. Evid. Based Complement. Alternat. Med. 2021, 6421122 (2021).
Gao, Y. et al. Cross-talk between Wnt/β-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS One 8(12), e82436 (2013).
Zhang, P. et al. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur. J. Pharmacol. 617(1–3), 130 (2009).
Fischer, V. & Haffner-Luntzer, M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol. 123, 14-21 (2022).
Peterson, W. J., Tachiki, K. H. & Yamaguchi, D. T. Serial passage of MC3T3-E1 cells down-regulates proliferation during osteogenesis in vitro. Cell Prolif. 37(5), 325–336 (2004).
Yin, Z. et al. miR-215-5p regulates osteoporosis development and osteogenic differentiation by targeting XIAP. BMC Musculoskelet. Disord. 23(1), 789 (2022).
Weiwei, Li. et al. Vitamin K2 stimulates MC3T3-E1 osteoblast differentiation and mineralization through autophagy induction. Mol. Med. Rep. 19, 3676–3684 (2019).
Shahrokh, L. et al. Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochim. Biophys. Acta Mol. Cell Res. 1868, 118926 (2021).
Liu, J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Sign. Transduct. Target. Ther. 7(1), 3 (2022).
Shah, K. & Kazi, J. U. Phosphorylation-dependent regulation of Wnt/beta-catenin signaling. Front. Oncol. 12, 858782 (2022).
Ge, X. & Zhou, G. Protective effects of naringin on glucocorticoid-induced osteoporosis through regulating the PI3K/Akt/mTOR signaling pathway. Am. J. Transl. Res. 13(6), 6330–6341 (2021).
Li, Y. et al. Ebselen rescues oxidative-stress-suppressed osteogenic differentiation of bone-marrow-derived mesenchymal stem cells via an anti-oxidant effect and the PI3K/Akt pathway. J. Trace Elem. Med. Biol. 55, 64–70 (2019).
Benisch, P. et al. The transcriptional profile of mesenchymal stem cell populations in primary osteoporosis is distinct and shows overexpression of osteogenic inhibitors. PLoS One 7(9), e45142 (2012).
Sherman, B. T. et al. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50(W1), W216–W221. https://doi.org/10.1093/nar/gkac194 (2022).
Kanehisa, M. et al. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51(D1), D587–D592 (2023).
Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13(11), 2498–2504 (2003).
Schrödinger, L., & DeLano, W. (2020). PyMOL. Retrieved from http://www.pymol.org/pymol
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 9(7), 671–675 (2012).
Acknowledgements
This study was funded by a grant from the Scientific Research Projects of Basic Scientific Research in Colleges and Universities Operating Expenses of Heilongjiang Province in 2023, China [2023-KYYWFMY-0005].
Author information
Authors and Affiliations
Contributions
H.W., YR.W, JY.Z., and Y.L. performed the experiments, interpreted the data and wrote the manuscript. J.L., YY.Z., and YX.M performed the experiments and interpreted the data. XF.Y. and H.W. designed the study and interpreted the data. XF.Y. and P.C. supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Liang, J., Wang, Y. et al. Exploring the effects of naringin on oxidative stress-impaired osteogenic differentiation via the Wnt/β-catenin and PI3K/Akt pathways. Sci Rep 14, 14047 (2024). https://doi.org/10.1038/s41598-024-64952-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64952-2
- Springer Nature Limited