Abstract
FN-doped carbon dots were synthesized using powdered leaves of Moringa oleifera L./Chromolaena odorata L./Tridax procumbens L./Tinospora cordifolia L./ and Lantana camara L., along with a precursor called 4,5-difluoro-1,2-benzenediamine (DFBD) and compared against the drug zaltoprofen derived carbon dots. They were assessed for their optical and structural characteristics using photoluminescence (optimal emission λ of 600 nm), vibrational (FTIR) spectroscopy (characteristic wave numbers of 1156 and 1269 cm−1 for C–F), as well as X-ray diffraction (XRD) (highest intensity at 27.56°) and high-resolution transmission electron microscopy (HR-TEM) (particles in the size range of 15–20 nm). Further, field emission scanning electron microscopy (FESEM) / energy dispersive spectroscopy (EDX) indicated FN do** of oval/oblong carbon dots. Membrane protection in percent is found to be 55.3 and 80.4 for FN-CDs and Z-FN-CDs respectively. The DPPH-free radical scavenging activity by FN-CDs was 69.4%, while with Z-FN-CDs, it was 54.2%. When tested on six bacterial strains (three each for gram-positive and gram-negative), the FN-CDs displayed a halo (ZOI) between 9 and 19 mm, whereas the Z-FN-CDs displayed a clearance zone between 9 and 17 mm. The FN-CDs showed significant emission-red-shift effects and demonstrated concentration-dependent biocompatibility and viability in neuroblastoma and beta-TC6-cell lines.
Similar content being viewed by others
Introduction
In recent years, advances in nanoscale technologies have led to the use of nanobiomaterials in medical applications. Fluorescent carbon nanoparticles were discovered by serendipity while purifying single-walled carbon nanotubes1. Carbon dots have numerous remarkable properties, such as solubility in aqueous and organic solvents, photoluminescence, high fluorescence, and stability2,3. CDs are synthesized by either “top-down”/ “step-wise refinement” (arc discharge, laser ablation, and acidic oxidation) or “bottom-up”/inductive (combustion, microwave, hydrothermal, and electrochemistry) approaches from a wide array of substrates4,5,6. The synthesis of carbon dots from plants Prosopis juliflora, Vachellia nilotica, Brassica oleracea, Centella asiatica, Annona squamosa, Azadirachta indica, and Mentha piperita has been attempted in recent times for applications in drug delivery, bioimaging, photothermal therapy, angiogenesis; biological potential such as antibacterial/anti-inflammatory/anti-oxidant properties; detection of metal ions (Fe3+), antibiotic (metronidazole), quantitation of hypochlorite, carbendazim (fungicide), and assay of dopamine7,8,9,10,11,12,13. Medicinal plants can be a suitable natural resource for synthesizing CDs with desired properties, by virtue of their bioactive composition. This is exemplified by the fluorescent hydrogel prepared using cationic carbon dots, acrylic acid and pectin, which showed powerful broad-spectrum antibacterial activity, which is 108.5 times more than that of other hydrogels14. Wang et al. found that CDs help in the migration of epithelial cells by epithelial-mesenchymal transition, decrease the inflammatory response and granulation tissue area, and thus contribute to the overall wound healing process15. Interestingly, carbon dots derived from diverse materials such as sugarcane, industrial waste and natural honey are finding potential applications in nonlinear optical devices, bioimaging, pharmaceutical and therapeutic applications16,17.
Heteroatom (nitrogen, sulphur, boron, phosphorus, and gadolinium) “Do**” is a method to enhance the photoluminiscent characteristics of carbon dots18,19,20,21,22,23,24. Fluorine is the preferred element because of its highest electronegativity and, hence, high absorptivity of adjacent electrons, resulting in the segregation of cationic and anionic charges25. The curative efficacy of drugs26, chemical firmness of proteins27 in biological systems, and phase alienation in polar/aqueous and non-polar/organic environments are all found to increase with grafted fluorine28,32,33, one-step ring-opening polymerization–dehydrative carbonization34, microwave35, hydrothermal36, and oxidative cutting techniques37. The antecedents of fluorine are melamine and ammonium fluoride31, 4,5-difluorobenzene-1,2-diamine32, tetrafluoroterephthalic acid33,34, polyethyleneimine (600 Da) and fluorinated diglycidyl ethers35, levofloxacin36, and fluorinated graphite37 in most cases. Well known applications of fluorine-doped CDs are in the areas of bacterial imaging, bio-imaging of cell lines like HEK 293, B16-F10, and detection of intracellular silver; efficacious gene transfection and biocompatibility in contrast to commercial reagents like lipofectamine 2000 and polyethylenimine (25 kDa), photodynamic therapy, and measurement of 4-nitrophenol31,32,33,34,35,36,37. Zaltoprofen, classified as a non-steroidal anti-inflammatory drug, is an analgesic propionic acid derivative with potent anti-inflammatory and anti-nociceptive properties. It acts as a cyclooxygenase inhibitor, decreases the generation of prostaglandin E2 (PGE2), and inhibits the bradykinin and lipoxygenase pathways of nociception38. However, non-selective inhibition of COX creates adverse effects such as peptic ulcers, platelet dysfunction and nephrotoxicity.
The bioactive phytoconstituents viz. kaempferol, scutellarein, isosakuranetin, rutin, coumaric acid, quercetin, gallic acid, niazinin, luteolin, and berberine of Moringa oleifera L., Chromolaena odorata L., Tridax procumbens L., Tinospora cordifolia L. and Lantana camara L. exhibit antipyretic, anti-inflammatory, antibacterial, hypoglycemic, immunomodulatory, and antitumor properties39,40,41,42,43 due to the presence of various bioactive compounds like flavonoids, polyphenols, alkaloids, quinines etc. Table S1 presents the results of 17 tests performed for preliminary phytochemical screening of the chosen plant leaf extracts. Extraction or Isolation of bioactives from these plant organs is possible, but it is tedious, time consuming, and requires carcinogenic solvents. A good substitute for this traditional method is the green synthesis of desired heteroatom-doped CDs from medicinal plant materials without the use of toxic solvents and a minimal purification protocol44. Heteroatom-doped CDs are prepared by do** the CDs in the respective precursor-solution(s). The incorporation of fluorine and nitrogen enhances the mechanical stability under repeated dynamic loading and piezoelectric properties in carbon dots that are essential requirements for their effective functioning as integral parts of biomedical devices employed in tissue regeneration and remodeling. Another significant benefit of heteroatom do** is the tuning of photoluminescence in CDs while enhancing their fluorescence45. Thus, the fabrication of heteroatom doped CDs from abundantly available medicinal plants with enhanced therapeutic properties in an eco-friendly, low-cost process is highly desirable.
The main medicinal uses of CDs at the moment are being studied by researchers. These uses include antibacterial, antioxidant, and anti-inflammatory properties, inadequacies in which are the primary cause of illnesses and diseases in humans3,46. The use of dietary supplements regularly as a part of traditional methods of treatment exposed their harmful effects on vital organs such as the liver, kidney and heart. Consequently, there was an imperative need for the development of alternatives; One such alternative identified was the synthesis of CDs from medicinal plant components, which have fewer accompanying side effects on living systems. Biomass valorization in recent years inspired the present study of FN-CD synthesis from medicinal plants (Moringa oleifera L., Chromolaena odorata L., Tridax procumbens L., Tinospora cordifolia L., Lantana camara) and the anti-inflammatory drug Zaltoprofen, employing DFBD as the depot for “fluorine”, and subsequent investigation of the optical, antioxidant, anti-inflammatory, and hypoglycemic properties of doped CDs together with their biocompatibility potential. A one-pot solvothermal-assisted green process was adopted for the synthesis of FN-CDs that emitted yellow fluorescence, and their emission wavelength showed a red shift by approximately 50 nm. FN-CDs derived from plant and Z-FN-CDs synthesized from zaltoprofen, demonstrated excellent biocompatibility when tested on neuroblastoma and beta-TC6-cell lines, reinstating their possible use for biomedical applications. At higher concentrations, they can be used as anticancer agents because of their evident antiproliferative and cytotoxic potential. To the best of our knowledge, this is the first FN-CD synthesized using the principles of a green and sustainable approach and taken forward for wound healing and cytotoxicity assessment. The entire synthesis process is fast, repeatable, and could easily be scaled up with excellent photoluminescence and cytocompatibility at lower concentrations, indicating their imminent importance in biomedical applications as therapeutic agents.
Results
Synthesis and characterization
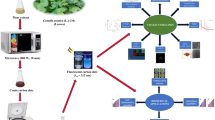
The biomolecules present in leaf powder are transformed into carbon dots by dehydration and carbonization and doped with F- and N-atoms using DFBD as the precursor agent. Thus, yellow-light-emitting-carbon-dots are formed after the solvothermal-process at 180 °C for 8 h. Similarly, ethyl-alcohol-soluble, zaltoprofen-derived carbon dots doped with F and N atoms are formed with the same precursor and under the same operating conditions as those used in the solvothermal process (Fig. 1).
Knowing the optical band gap from UV absorbance data offers the advantage of facilitating the fabrication of biocompatible and degradable materials in biomedical applications. Energy band gaps between 1 and 2.5 eV, is considered crucial for electronic device manufacturing. Additionally, optical band gaps play a significant role in characterizing carbonaceous materials, aiding in the identification of different structures and their degree of aromatization, which is vital in various biomedical applications. In the present study, Tauc’s method was employed to calculate the direct optical band gap (Fig. S2a) and the same was shown to be between 3.79 and 4.51 eV for FN-CDs, while it was between 3.92 and 4.24 eV for Z-FN-CDs (Fig. S2b).
FESEM analysis confirmed the oval/oblong shape of FN-CDs derived from plants and Z-FN-CDs derived from the commercial drug Zaltoprofen (Fig. 2a and c). EDX analyses confirmed surface functional groups and FN do** in the carbon dots, with low intensity signals for fluorine and nitrogen (Fig. 2b, d; Table 1) as compared to carbon and oxygen. Data from the HR-TEM images taken at a resolution of 20 nm and 10 nm are presented in Fig. S1a and b and the images at 2 nm resolution (Fig. 3a and b) were analyzed by image J software for determination of particle sizes. The analyses suggest that the size range of the particles, derived from both the source materials, is predominantly between 15 and 20 nm (Fig. 3a(i) and b(i)). However, significantly higher number of FN-CDs measured 15 nm while for Z-FN-CDs, the difference in the numbers of particles measuring 15 and 20 nm was relatively less. The calculated interplanar distances (Gaton digital micrograph) were similar with 0.23 and 0.2 nm for the FN-CDs and Z-FN-CDs, respectively (Fig. 3a(ii) & b(ii)). The selected area electron diffractograms for the FN-CDs and Z-FN-CDs are presented in Fig. 3c and d.
The chemical structure of FN-CDs was characterized using an FTIR-Spectrum (Fig. 4a). The O–H and N–H stretching vibrations are shown by the pulse amplitudes (PAs) at 2851, 2921, 3135, and 3650 cm−1, whereas the carboxyl groups are indicated by the PAs at 1735 and 1019 cm−1. The existence of a C=O functional group is indicated by the peak at 1650 cm−147, and the presence of aromatic rings is indicated by the PAs at 1624 and 1483 cm−1, which may be attributed to the vibration of conjugate C=C bonds. The –CH2-moiety is indicated by the peak at 1364 cm−1. C–F-Bonding is responsible for C–F-Vibrations, particularly the F-aryl-mode at 1156 and 1269 cm−1. The Vibrations caused by C–N-Stretching are responsible for the PAs at 1400 and 1200 cm−148. Z-FN-CDs’ FTIR-Spectra revealed PAs at 2933 and 3063 cm−1, which corresponded to O–H and N–H stretching vibrations; 1717 and 1041 cm−1, which were associated with carboxyl groups; and 1647 cm−1, which was associated with the C=O functional group47. It is possible to attribute the PAs at 1636 and 1473 cm−1 to the conjugate C=C-bond-vibrations that characterize aromatic rings. Similar to FN-CDs, the C–F bonding is responsible for the C–F vibrations (F-aryl mode) at 1156 and 1266 cm−1. The peak at 1364 cm−1 represents the − CH2-moiety, and the C–F vibrations (F-aryl mode) at 1156 and 1266 cm−1 are attributed to C–F bonding, as in the case of FN-CDs (Fig. 3a).
(a) FTIR spectra of FN-CDs and Z-FN-CDs with wave numbers corresponding to specific functional groups and associated bond stretching/vibrations; (b) X-ray diffractogram of FN-CDs, (c) Z-FN-CDs depicting prominent peaks characteristic for carbon dots with markings of 2θ, hkl indices and d-spacing as obtained from Match 4 software.
The X-ray diffractogram of FN-CDs showed a tiny peak at 43°, and strong pulse amplitudes (PAs) at 2θ-values of 15.4°, 27. 56°, and 31. 6° demonstrate their crystalline structure (JCPDS 26–1076; Fig. 4b). The presence of functional groups and partial graphitization of the doped carbon dots resulted in less pronounced PAs at 2θ-values of 19.1° and 42.5° in the Z-FN-CD-X-ray diffractogram (Fig. 4c). The XRD data were utilized to understand the crystal quality characteristics viz. lattice constant, strain and dislocation density for both FN-CDs and Z-FN-CDs. The calculated values for the three parameters were 10.2 Å, 69.6, 22.9 nm−2 respectively for FN-CDs while the same for Z-FN-CDs were 7.4 Å, 152.2 and 62.1 nm−2 in the order. In general, decrease in lattice constant means the electrons are more tightly bound to the atom, and hence require more energy to remove, leading to an increased band gap. From the results presented, it is evident that Z-FN-CDs have tightly bound electrons. Further, low strain and dislocation densities for FN-CDs are important indicators of their biomedical applications, predominantly as potential therapeutic agents, drug delivery, bioimaging and biocompatibility.
An ideal zeta potential value for biomaterial derived carbon dots is positive and it correlates with enhanced biological functions like adherence in wound healing and tissue regeneration because of their biocompatibility and ability to mimic extracellular matrix (ECM), and enhanced stability of emulsions and suspensions due to large positive value induced repulsions between particles. The assessment of zeta potential for FN-CDs showed a sharp peak at 19.4 mV (Fig. 5a), while Z-FN-CDs showed a maximum peak intensity at 0.3 mV (Fig. 5b). These results are suggestive of possible impregnation of FN-CDs in nanofibrous wound dressings and in the formulation of pharmaceutical liquid dosage forms like suspensions and emulsions.
Optical properties
FN-CDs and Z-FN-CDs’ optical characteristics were examined by UV–vis spectroscopy and photoluminescence experiments48. The FN-CDs had three UV–vis-absorption PAs at 260, 380 and 440 nm, as seen in Fig. 6a. These PAs may be associated with the C=N or C=C bonds’ π–π* transition. Furthermore, a wide absorption band spanning from 400 to 500 nm was noted; However, its maximum absorption peak was located at 440 nm and was attributed to the C=O group’s n–π* transition49,50. In contrast, two UV–vis-absorption PAs at 240 and 330 nm are present in the Z-FN-CDs (Fig. 6b). Under a UV-Lamp, the FN-CDs and Z-FN-CDs displayed fluorescence (yellow colour) at 365 nm with overlap** excitation and emission spectra (Fig. 6a, b).
By progressively increasing the excitation wavelengths from 300 to 580 nm (at 20 nm intervals), the photoluminescence properties of FN-CDs and Z-FN-CDs were determined. The corresponding emission wavelengths are shown in Fig. 6c and d, respectively. The emission-wavelength of the FN-CDs and Z-FN-CDs ranged from 550 to 600 nm, depending on the excitation-wavelength (300–580 nm). Z-FN-CDs showed the peak at 550 nm against an excitation wavelength of 460 nm, while FN-CDs showed their optimal/maximum emission peak at 600 nm for an excitation wavelength of 560 nm.
Biological functions
DPPH scavenging
Ascorbic Acid, a well-known powerful antioxidant, was used as a standard for measuring the depletion of reactive oxygen species. This anti-oxidative ability of ascorbic acid increased from 34.45 to 86.43% for the chosen concentrations, whereas the same for FN-CDs ranged between 29.49 and 69.39% and for Z-FN-CDs, it was between 28.27 and 54.24% (Fig. 7a). IC50 values for ascorbic acid, FN-CDs and Z-FN-CDs obtained from the DPPH-assay were 11.41 μg/mL, 15.6 μg/mL and 24.03 μg/mL, respectively (Table 1). Thus, the free radical scavenging ability of DPPH reflects its ability to accept hydrogen from the molecule of ascorbic acid and the surface functional groups of FN-CDs and Z-FN-CDs to form a stable DPPH-H complex (Fig. 7b)51,52,53. Scavenging of the DPPH-radical by FN-CDs and Z-FN-CDs in the concentration range between 5 and 25 μg/mL is inferred from the decreasing photometric absorbance values measured at 517 nm.
(a) DPPH foraging activity of FN-CDs and Z-FN-CDs in percent with ascorbic acid standard; (b) illustrative explanation for the mechanism of action of FN-doped carbon dots on DPPH free radical neutralization; (c) hypoglycemic activity of FN-CDs and Z-FN-CDs in terms of the enzyme amylase inhibition with acarbose standard; (d) anti-inflammatory potential in terms of human RBC (HRBC) membrane protection effected by FN-CDs and Z-FN-CDs; (e) mechanism of anti-inflammatory action of CDs in comparison with natural cells of human body.
Hypoglycemic activity
One of the methods used to control hyperglycemia is the inhibition of amylase. The antidiabetic potential of FN-CDs and Z-FN-CDs was determined by estimating the amylase-inhibition effect. Acarbose is a commercial amylase inhibitor and is used as a standard to compare amylase inhibition by FN-CDs and Z-FN-CDs. The percentage-inhibition-effect of FN-CDs and Z-FN-CDs was compared with that of acarbose (Fig. 7c), and the IC50values are tabulated in Table 1. The percentage inhibition caused by FN-CDs, Z-FN-CDs and acarbose increased from 17.94 to 72.64%, 5.9 to 38.44%, and 49.94 to 82.52%, respectively, with an increase in concentration from 50–250 μg/mL. This demonstrates that FN-CDs and Z-FN-CDs, incorporated in medications, can have considerable hypoglycemic effects.
Anti-inflammatory activity
Experimental investigation of RBC membrane stability is ideal for evaluating anti-inflammatory properties and is easy to execute. Hypotonically-induced hemolysis of human red blood cells (HRBCs) was adopted in the study. Figure 7d depicts the effect of FN-CDs, Z-FN-CDs and standard diclofenac on the HRBC-membrane and the corresponding statistical data are presented in Table 1. The reference drug ‘diclofenac’ exhibited membrane protection to the extent of 32.9–84.5%, while the percentage of protection increased from 16 to 55% and 23 to 80% with the increase in concentration from 5 to 25 μg/mL for FN-CDs and Z-FN-CDs, respectively. The IC50 values for the diclofenac standard, FN-CDs, and Z-FN-CDs were 11 μg/mL, 22.2 μg/mL and 13.3 μg/mL, respectively. The anti-inflammatory effect of carbon dots derived from plants is represented in Table 2. Our results are in approximation with the findings of hemolytic analysis with MgO nanostructures54 and CdO–NiO composites55.
Antibacterial activity
The carbon dots were tested against three gram-positive bacteria (methicillin-resistant S. aureus (MRSA), S. epidermidis, and S. hemolyticus) and three gram-negative bacteria: E. coli, K. pneumoniae, and Pseudomonas sps. FN-CDs and Z-FN-CDs showed maximum ZOI for S. epidermidis (Fig. 8a, b) and MRSA. From the zones of inhibition (ZOI) obtained, it is obvious that FN-CDs and Z-FN-CDs exhibited a linear increase in antibacterial activity with an increase in the concentrations of FN-CDs and Z-FN-CDs tested on Gram + ve/ − ve bacteria (Fig. 8c and d), a reflection of their dose-dependent activity. The ZOI ranged in between 10 and 19 mm for FN-CDs (Table 3), whereas the same for Z-FN-CDs is 10 and 17 mm (Table 4). At 500 μg/well of the test substance, S. epidermidis was more effectively inhibited by both FN-CDs and Z-FN-CDs among the three gram-positive tested strains, followed by methicillin-resistant S. aureus (MRSA) and S. hemolyticus. Among gram-negative bacteria, E. coli and K. pneumoniae were better inhibited, followed by Pseudomonas sps, with the tested dose of 500 μg/well of FN-CDs (Fig. S3). Among the three studied gram-negative bacteria with Z-FN-CDs, Pseudomonas sps. is best inhibited, followed by E. coli and K. pneumoniae. The results of our study corroborate with the zones of clearance obtained with the carbon quantum dots56 prepared from Manihot esculenta waste peels, silver nanoparticles synthesized from Datura metel L.57, and nanocomposites of Y3+ and Sm3+ mixed metal oxides58 as well as ceria (Cerium IV-oxide) based nanomaterials59.
(a) Antibacterial activity of FN-CDs and Z-FN-CDs against S. epidermidis; (b) Schematic sketch depicting bacterial membrane/cell wall disruption by the carbon dots; (c) zones of Inhibition (ZOI) obtained for the tested human clinical isolates (mm) at different concentrations of FN-CDs; (d) zones of Inhibition (ZOI) obtained for the tested human clinical isolates (mm) at different concentrations of Z-FN-CDs.
Cell viability
Neuroblastoma and beta-TC6-cell lines were treated with different concentrations (15–500 μg/mL) of FN-CDs, and cell viability was measured by the MTT-assay after 24 h of incubation (Fig. 9a). The different concentrations of FN-CDs (15, 31, 62, 125, 250 and 500 μg/mL) showed a decrease in the percent-cell-viability in the order of 91.68, 86.67, 80.98, 71.39, 61.29 and 49.74 respectively for neuroblastoma-cells, with reference to the untreated control-cells. The same concentrations of FN-CDs for beta TC6-cells showed a similar trend with viability values of 74.65, 67.7, 60.96, 54.3, 47.5 and 44.7, respectively, in comparison to the untreated control cells (Fig. 9b). Of the two cell lines tested, the neuroblastoma-cells demonstrated more than 80% viability only up to 62 μg/mL concentration of FN-CDs (Fig. 9c), and beyond this, the viability dropped steeply. This trend could be positively manipulated for effecting the desired therapeutic function of either proliferation for tissue regeneration and/or inducing cell death, as required in malignancy, just by varying the concentrations.
Discussion
Carbon dots were prepared by solvothermal process using Moringa oleifera L., Chromolaena odorata L., Tridax procumbens L., Tinospora cordifolia L. and Lantana camara L. leaf powders. The leaves containing cellulose, proteins, phenols, and falvonoids act as natural and remarkable green precursors for the carbon dots’ production. At 180 °C, the cellulose is hydrolyzed, phenols are degraded, and proteins and flavonoids are destabilized. DFBD is used as a precursor for the synthesis of carbon dots because of the high stability of fluorine in aromatic rings and the characteristics that the amino groups impart to the N-bearing units under solvothermal conditions, including positive charge localization, Schiff-base-Fragment, and N-heterocycle. The presence of C–F- and C–N-Bonds in the structure of carbon dots suggests the presence of F and N. The organic components in plant-leaf-extract provide the carboxyl- and hydroxyl-functional groups seen in the carbon-dots.
The X-ray diffractogram of FN-CDs revealed a crystalline structure with strong pulse amplitudes (PAs) at 2θ-values of 15.4°, 27.56°, and 31.6°. Z-FN-CDs showed less pronounced PAs at 19.1° and 42.5° (JCPDS 26-1076). XRD data revealed lattice constant, strain, and dislocation density for both FN-CDs and Z-FN-CDs. Low strain and dislocation densities of FN-CDs indicate their potential biomedical applications. An ideal zeta potential value for biomaterial-derived carbon dots correlates with enhanced biological functions like wound healing and tissue regeneration. FN-CDs showed a sharp peak at 19.4 mV, while Z-FN-CDs showed maximum peak intensity at 0.3 mV.
DPPH is a reliable, simple, and well-established method for estimating antioxidant activity. The free-radical-scavenging-ability prevents damage caused by the generation of free-radicals and maintains proper cellular function. In the presence of antioxidants, the purple-colored DPPH-Solution changes to the yellow-colored DPPH-H-Solution. The capacity of an antioxidant is deduced from its ability to donate hydrogen-ions51,52,53. Enzymatic antioxidants, exemplified by peroxidase, ascorbate peroxidase, and catalase, remove reactive species by transforming the oxidation products to water. These antioxidants are released from cells cultivated in a medium containing the cofactors copper, zinc, and manganese. The second class of non-enzymatic antioxidants includes vitamins, polyphenols, phenolic acids, flavonoids, ascorbic acid and glutathione; these compounds stop oxidation by inhibiting ROS-chain reactions60. DPPH (2,2-diphenyl-1-picrylhydrazyl) is a persistent free radical with a purple hue, and its assay is a quick, simple, and affordable way to assess the radical scavenging activity of non-enzymatic antioxidants. Carbon dots synthesized from plants are categorized as non-enzymatic radical scavengers61.
Carbon dots scavenge reactive species of nitrogen and hydroxyls (·OH), which otherwise impair cellular processes62. The surface of carbon dots releases hydrogen, which is then picked up by the DPPH-free radical, which has core nitrogen and two pairs of non-bonding electrons embraced by three benzene rings. While the unpaired electrons on the CDs are shifted through chemical bond rearrangement or resonance in aromatic domains, the presence of amino (–NH2, –NH–), carboxyl (–COOH), and hydroxyl (–OH) groups that supply hydrogen facilitates this reduction pathway of DPPH· to DPPH-H. ·OH-Radicals harm biomolecules including lipids, proteins, and nucleic acids through oxidative damage, and they are the prime cause of stress in biological units. Carbon dots can participate in electron transfer reactions with these ·OH-radicals and eliminate them by transformation into less reactive molecules through electron transfer61. The effectiveness of carbon dots in neutralizing many ·OH-radicals is reportedly increased by redox-recycling63,64. Furthermore, singlet-oxygen-molecules (ROS-radicals), which are responsible for intense oxidative damage, are neutralized by interaction with the excited states of carbon dots63,64. The antioxidant capabilities of C-dots are determined from their excited-state quenching62,65.
Amylase breaks down the starch into maltose (disaccharide) and glucose (monosaccharide) units. Zingiberis carbonisata-based carbon dots can reduce blood-glucose-levels in diabetic mice. In addition, these dots decrease the levels of inflammatory cytokines and suppress protein expression66. Carbon dots made from Artemisiae Argyi Folium (AAF) carbonisata have anti-inflammatory properties because they inhibit the expression of inflammatory mediators and lower blood-glucose levels in mice67.
Anti-inflammatory drugs used to combat inflammation stabilize the lysosome membrane and prevent the release of acidic lysosomal enzymes in the cytosol. The bioactive compounds and functional groups present on carbon dots are presumed to inhibit the lysis of the RBC membrane. The FN-CD-loaded HRBCs are protected from being lysed by the induced hypotonic environment, indicating that the membranes of the RBCs are stabilized by the doped-carbon-dots. The carbon dots derived from plants exhibit an anti-inflammatory effect due to their ability to forage reactive radicals (Fig. 7e) and interact with components of signaling pathways for inflammation by down regulating the pro-inflammatory mediators like “TNF-α, IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-12, and IFN-γ receptors” and empower the immune system68,69,70. The carbon dots synthesized from broccoli using the hydrothermal method were tested in zebrafish for their anti-inflammatory activity and were found to reduce the expression of “TNF-α” and “IL-6”. The expressions of glutathione peroxidase (GPX-4) and superoxide dismutase (SOD) are upregulated, thus increasing the antioxidant activity and reducing inflammation7. The carbon dots derived from mulberry silkworm cocoon carbonisata (MSCC) showed anti-inflammatory activity in a lipopolysaccharide-induced inflammation model, which closely resembles sepsis in humans71. Cerium-doped carbon nanodots are used as anti-inflammatory agents in mice for wound healing72.
The amines and amides present on the surface of carbon dots were inferred to affect the antimicrobial activity of dots because of the electrostatic interactions between the protonated groups and lipids present in the bacterial cell membrane. Furthermore, the F− and NH2+ groups on the surfaces of doped CDs react with the cell wall/cell membrane components of the bacteria and affect their lysis. The rise in multidrug-resistant (MDR) bacterial wound-infections to available antibiotics, accompanied by a continuous decline in antibiotic development, is a major global issue. Therefore, there is a need to synthesize the agents that act against the MDR-bacterial strains. The first step of interaction between the antimicrobial agents, FN-CDs and Z-FN-CDs in this study, and bacteria is direct, through electrostatic action and/or chemical conjugations. The study results are in corroboration with the two proposed mechanisms that exist for CDs to attach to the bacterial cell membrane and cause physical or mechanical damage, viz. the rupture of the bacterial cell wall, allowing the CDs to enter the internal membranes, the membrane collapse due to loss of cytoplasmic fluids and electrolytes73, and the electrostatic interactions for surface adherence74. The amine groups present on CDs denatures the DNA, resulting in apoptosis of the cell75. ** and their applications for red cell imaging and sensitive intracellular Ag+ detection. J. Phys. Chem. C 121, 26558–26565 (2017)." href="/article/10.1038/s41598-024-63700-w#ref-CR32" id="ref-link-section-d212296508e3571">32.
Characterization of the carbon-dots
The synthesized carbon dots were observed under a UV-Lamp (Analytik Jena, CA, USA) in Gel-Doc-Equipment and noted their fluorescence color. The carbon dots were analytically characterized by UV–vis (UV2000U, Labindia), fluorescence (Horiba, JobinVyon), Fourier transform infrared (FTIR; Shimadzu, Miracle 10), and X-ray diffraction (Rigaku diffractometer Ultima IV 2036E202) methods. The zeta potential was evaluated using a particle-size-analyzer (Horiba, SZ-100). While the shape and form of the carbon dots was observed by high resolution transmission microscopy (HRTEM, 200 kV), Image J and Match 4 soft wares were used to calculate the diameter, lattice constant, strain, dislocation density and d-spacing of the carbon dots. Cell survival was tested by MTT-Assay and estimated with the aid of a microplate reader (Bio-Rad, USA).
Carbon Dots’ DPPH-radical forage activity was assessed through the application of a slightly altered version of Hsu et al.78. 5 mL of DI-Water, 3 mL of 0. 3 mM DPPH in methyl-alcohol, and the designated doses of FN-CDs and Z-FN-CDs in micrograms per milliliter of 5, 10, 15, 20, and 25 were added to experimental tubes. These were then gently vortexed and left in an aphotic environment at ambient temperature for half an hour. In parallel with the experimental tubes, a control was set up with the exclusion of test material. As positive controls, various aliquots of ascorbic acid in the concentrations corroborating with the test samples were employed for the conduct of the assay. The Reduction of DPPH was detected by a visible photometer at 517 nm (Labindia, Model: UV3200).
The absorbance measurements of the control and test/standard are Ac and At respectively. The concentration of FN-CDs/Z-FN-CDs (μg/mL) was plotted against the percentage of DPPH-scavenging-activity on the graph. From the regression equation, the IC50 was calculated and ANOVA was performed.
Hypoglycemic activity
The “amylase inhibition” was estimated using the method proposed by Bernfeld, with minor changes79. Different concentrations in micrograms per milliliter of FN-CDs/Z-FN-CDs, viz. 50–250, were added to 1 mL of buffer containing KH2PO4 and NaOH (pH 11.0), 1 mL of 1% aqueous starch, and 1 mL of human salivary amylase and incubated at 20 °C for 5 min. After adding 2 mL of di-nitro salicylic acid (DNS) reagent to every experimental tube, the tubes were placed at 70 °C in a hot-water-bath for 10 min, followed by cooling with flowing tap-water. Lastly, 10 mL of water were mixed with the brown reduction product that had been collected in the experimental tubes, and an optical spectrophotometer was used to measure the OD-values at 540 nm. Everything was prepared for a blank, with the exception of the amylase-enzyme. A control was placed without the test sample (carbon dots), which represents 100% enzyme activity. A commercial antidiabetic drug, acarbose (50, 100, 150, 200, and 250 μg), was used as a positive control. One milliliter of starch (1%) dissolved in phosphate buffer (0.02 M, pH 6.9) and one milliliter of human salivary amylase were added to the acarbose solution, and it was then incubated at 20 °C for five minutes. After adding 2 mL of DNS-reagent, the previously described procedures for the doped CDs were then carried out. The percent inhibition of amylase by CDs and acarbose is expressed as follows:
The optical density values of the test and the control samples are denoted by Ac and At, respectively.
Membrane stability
In vitro testing of Carbon dots’ anti-inflammatory properties was done using stabilization of Human Red Blood Cell membranes80. Heparinized, purple-capped ethylene diamine tetra acetic acid (EDTA) tubes were used to hold the 2 mL of blood that were taken from each of the healthy volunteers, who had not taken any medicine in the previous two weeks, with their informed agreement. After centrifuging the test sample for 15 min at 3000 revolutions per minute to separate the RBC and plasma, the recovered RBCs were repeatedly washed with an isotonic solution of NaCl (0.9%) and then centrifuged again. Using the same isotonic NaCl solution, the collected centrifuge was diluted to a 10% solution (v/v). Informed consent was obtained from all human subjects who participated as volunteers in the study, as per institutional ethics committee (IEC) guidelines.
Induced hemolysis
To various concentrations of FN-CDs/Z-FN-CDs (5–25 μg/mL), 0.5 mL of human RBC (HRBC) suspension, 2 mL of 0.2% NaCl (hyposaline) and 2 mL of 0.15 mM phosphate buffer at pH 7.4 were added. For the control, deionized (DI) water was added instead of hypotonic NaCl. Diclofenac was used as the standard reference. The Analysis tubes were maintained at 37 °C for 30 min and centrifuged at 3000 rpm for 15 min. Hemoglobin content in the supernatant was estimated spectrophotometrically (λ (nm) = 560). The percentage hemolysis was determined from the expression:
Antibacterial-activity
The antibacterial capacity of FN-CDs/Z-FN-CDs was tested against pathogenic human clinical isolates that included methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, and Staphylococcus hemolyticus under gram positive, while Escherichia coli, Klebsiella pneumoniae, Pseudomonas sps were under gram negative. They were procured from the microbiology department of the local multispecialty hospital and medical college, following the institutional ethical committee guidelines. Nutrient-agar-medium was used as the cultivation medium for the bacterial strains. After 2 passes, 0.6 OD bacterial cultures were used for antibacterial activity evaluation. The clinical isolates were cultured in peptone water (Hi-Media) medium under aerobic conditions at 37 °C for 24 h. With the help of a sterile corkborer, six wells of 6 mm depth were punched. Then, FN-CDs/Z-FN-CDs in DMSO (50 mg/mL) were taken in volumes of 10, 25, 50, 75, and 100 μl, and added in a clockwise direction in the order of wells labeled from 1 to 5. A 50 μl negative control consisting of dimethyl sulfoxide was added in the sixth well. A 75/10 mcg ticarcillin/clavulanic acid (TCC) antibiotic disk was placed in the center of the plate, incubated at 37 °C overnight and recorded the zones of clearance.
Biocompatibility testing with cell lines
Neuroblastoma and beta-TC6-cells were acquired from the National Centre for Cell Science (NCCS), Pune, India. Hams’ F12K-medium for neuroblastoma-cells, DMEM (Dulbecco’s modified Eagle-medium) and F-12 (Hams’ F-12 nutrient mixture) in 1:1 proportion for beta-TC6-cells, were added with 10% FBS, and antibiotics of the classes aminoglycoside (streptomycin) and β-lactam (penicillin). A final concentration of 1 × from a 100% stock of either of the media was used in strict aseptic conditions throughout the experimentation. The cells were treated with Trypsin–EDTA after the attainment of fluent growth. Then 106 cells of each of the chosen cell lines were seeded in standard culture plates of 96 wells and kept in an incubator with atmospheric conditions of 95% humidity and 5% carbon dioxide. The biocompatibility test was performed in triplicate. Different concentrations of FN-CDs, namely 15 μg, 31 μg, 62 μg, 125 μg, 250 μg, and 500 μg, were applied to a 100 μl volume of cells and incubated with 50 μl of (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) for three hours at 37 °C. After incubation, to each test tube phosphate-buffer saline of 200 μl was added, and any residual MTT, if found, was carefully removed. For solubilization, 200 μl of acid-propanol were then added and left in the dark for the entire night. A phase-contrast-microscope was used to view the cells, while a microplate reader read the absorbance at 570 nm. After 24 h, the absorbance of the control-cells (those not receiving treatment) was fixed at 100% Viability, and the percentage of vital-cells in the other treatment-groups was determined using the formula.
Data availability
The raw data of the current study are compiled and provided as a supplementary file.
References
Xu, X. et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 126(40), 12736–12737 (2004).
Zhao, P. & Zhu, L. Dispersibility of carbon dots in aqueous and/or organic solvents. Chem. Commun. 54, 5401–5406 (2018).
Gedda, G. et al. Green synthesis of multi-functional carbon dots from medicinal plant leaves for antimicrobial, antioxidant, and bioimaging applications. Sci. Rep. 13(1), 6371 (2023).
Wang, X., Feng, Y., Dong, P. & Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic Application. Front. Chem. 7, 671 (2019).
Prathap, N. et al. Prosopis juliflora hydrothermal synthesis of high fluorescent carbon dots and its antibacterial and bioimaging applications. Sci. Rep. 13, 9676 (2023).
Al Salem, H. S., Binkadem, M. S., Al-Goul, S. T. & Abdel-Lateef, M. A. Synthesis of green emitted carbon dots from Vachellia nilotica and utilizing its extract as a red emitted fluorescence reagent: Applying for visual and spectroscopic detection of iron (III). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 295, 122616 (2023).
Deng, W. et al. Hydrothermally derived green carbon dots from broccoli water extracts: Decreased toxicity, enhanced free-radical scavenging, and anti-inflammatory performance. ACS Biomater. Sci. Eng. 9(30), 1307–1319 (2023).
Thokchom, B., Bhavi, S. M., Abbigeri, M. B., Shettar, A. K. & Yarajarla, R. B. Green synthesis, characterization and biomedical applications of Centella asiatica-derived carbon dots. Carbon Lett. 33(4), 1057–1071 (2023).
Vibhute, A. et al. Green synthesis of fluorescent carbon dots from annona squamosal leaves: Optical and structural properties with bactericidal, anti-inflammatory, anti-angiogenesis applications. J. Fluoresc. 33(4), 1619–1629 (2023).
Raveendran, V. & Kizhakayil, R. N. Fluorescent carbon dots as biosensor, green reductant, and biomarker. ACS Omega 6(36), 23475–23484 (2021).
Lo, K. M., Lin, Y. S., Liou, J. W., Chiu, T. C. & Hu, C. C. Electrochemically synthesized green fluorescent carbon dots for quantitation of hypochlorite and carbendazim. J. Food Drug Anal. 31(2), 244–253 (2023).
Qi, H. et al. Novel N-doped carbon dots derived from citric acid and urea: fluorescent sensing for determination of metronidazole and cytotoxicity studies. RSC Adv. 13(4), 2663–2671 (2023).
Chen, J. et al. Multi-applications of carbon dots and polydopamine-coated carbon dots for Fe3+ detection, bioimaging, dopamine assay and photothermal therapy. Discov. Nano 18, 30 (2023).
Cui, F. et al. Carbon dots-releasing hydrogels with antibacterial activity, high biocompatibility, and fluorescence performance as candidate materials for wound healing. J. Hazard. Mater. 406, 124330 (2021).
Wang, Z. et al. Carbon dots induce epithelial-mesenchymal transition for promoting cutaneous wound healing via activation of TGF-b/p38/snail pathway. Adv. Funct. Mater. 30, 2004886 (2020).
Pandiyan, S. et al. Biocompatible carbon quantum dots derived from sugarcane industrial wastes for effective nonlinear optical behavior and antimicrobial activity applications. ACS Omega 5(47), 30363–30372. https://doi.org/10.1021/acsomega.0c03290 (2020).
Surendran, P. et al. Bioinspired fluorescence carbon quantum dots extracted from natural honey. Efficient material for photonic and antibacterial applications. Nano-Struct. Nano-Objects 24, 100589 (2020).
Zhang, Q. et al. Preparation of one-emission nitrogen-fluorine-doped carbon quantum dots and their applications in environmental water samples and living cells for ClO- detection and imaging. J. Anal. Methods Chem. 7515979, 9 (2023).
Magdy, G., Ebrahim, S., Belal, F., El-Domany, R. A. & Abdel-Megied, A. M. Sulfur and nitrogen co-doped carbon quantum dots as fluorescent probes for the determination of some pharmaceutically-important nitro compounds. Sci. Rep. 13, 5502 (2023).
Yu, F. et al. Nitrogen and phosphorus co-doped carbon dots for the growth promotion of water spinach. Symmetry 15(8), 1532 (2023).
Cao, F. J., Hou, X., Wang, K. F., **, T. Z. & Feng, H. Facile synthesis of phosphorus and nitrogen co-doped carbon dots with excellent fluorescence emission towards cellular imaging. RSC Adv. 13, 21088–21095 (2023).
Mohandoss, S. et al. Nitrogen, sulfur, and phosphorus co-doped carbon dots-based ratiometric chemosensor for highly selective sequential detection of Al3+ and Fe3+ ions in logic gate, cell imaging, and real sample analysis. Chemosphere 313, 137444 (2023).
Xu, J. et al. Green one-step synthesis of boron and nitrogen co-doped carbon dots based on inner filter effect as fluorescent nanosensors for determination of Fe3+. Ceram. Int. 49(5), 7546–7555 (2023).
Fang, Y. et al. Facile synthesis of pH-responsive gadolinium(III)-doped carbon nanodots with red fluorescence and magnetic resonance properties for dual-readout logic gate operations. Carbon 166, 265–272 (2020).
Sun, W. C., Gee, K. R., Klaubert, D. H. & Haugland, R. P. Synthesis of fluorinated fluoresceins. J. Org. Chem. 62(19), 6469–6475 (1997).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Buer, B. C., Meagher, J. L., Stuckey, J. A. & Marsh, E. N. Structural basis for the enhanced stability of highly fluorinated proteins. Proc. Natl. Acad. Sci. 109(13), 4810–4815 (2012).
Horvath, I. T. & Rabai, J. Facile catalyst separation without water: fluorous biphase hydroformylation of olefins. Science 266(1582), 72–75 (1994).
**ong, S. D. et al. Cationic fluorine-containing amphiphilic graft copolymers as DNA carriers. Biomaterials 31(9), 2673–2685 (2010).
Percec, V. et al. Self-organization of supramolecular helical dendrimers into complex electronic materials. Nature 419(6095), 384–387 (2002).
Wang, N. et al. Fluorine-doped carbon nitride quantum dots: Ethylene glycol-assisted synthesis, fluorescent properties, and their application for bacterial imaging. Carbon 109, 141–148 (2016).
Zuo, G. et al. Large emission red-shift of carbon dots by fluorine do** and their applications for red cell imaging and sensitive intracellular Ag+ detection. J. Phys. Chem. C 121, 26558–26565 (2017).
Zuo, G. et al. Fluorine doped cationic carbon dots for efficient gene delivery. ACS Appl. Nano Mater. 1(5), 2376–2385 (2018).
Feng, S. et al. A novel application of fluorine doped carbon dots combining vortex assisted liquid-liquid micro extraction for determination of 4- nitrophenol with spectrofluorimetric method. J. Fluoresc. 29(5), 1133–1141 (2019).
Luo, T. Y. et al. Photoluminescent F-doped carbon dots prepared by ring opening reaction for gene delivery and cell imaging. RSC Adv. 8, 6053–6062 (2018).
Jiang, L. et al. Photoactivated fluorescence enhancement in F, N-doped carbon dots with piezochromic behavior. Angew. Chem. Int. Ed. 59, 9986 (2019).
Li, Z. et al. Fluorine-containing graphene quantum dots with a high singlet oxygen generation applied for photodynamic therapy. J. Mater. Chem. B 8, 2598–2606 (2020).
Li, L., Ma, P., Cao, Y., Tao, L. & Tao, Y. Single-dose and multiple-dose pharmacokinetics of zaltoprofen after oral administration in healthy chinese volunteers. J. Biomed. Res. 25(1), 56–62 (2011).
Chiș, A. Bioactive compounds in Moringa oleifera: Mechanisms of action, focus on their anti-inflammatory properties. Plants 13(1), 20 (2024).
Kota, S., Pradeep, D., Sajja, R. & Anantha, R. Phytoconstituents of Chromolaena odorata (L.) leaf extract for the synthesis of copper oxide/copper nanoparticles and evaluation of their biological potential in wound healing. Trends Phytochem. Res. 7(3), 186–206 (2023).
Varsharani, V. I., Pravin, C. M. & Sushma, R. K. Phytochemistry and pharmacological aspects of Tridax procumbens (L.): A systematic and comprehensive review. Phytomed. Plus 2(1), 1001994 (2022).
Ved, A., Arsi, T., Prakash, O. & Gupta, A. A review on phytochemistry and pharmacological activity of Lantana camara linn. Int. J. Pharm. Sci. Res. 9(1), 37–43 (2018).
Ahsan, R., Mishra, A., Badar, B., Owais, M. & Mishra, V. Therapeutic Application, phytoactives and pharmacology of Tinospora cordifolia: An evocative review. Chin. J. Integr. Med. 29(6), 549–555 (2023).
Kang, C., Huang, Y., Yang, H., Yan, X. F. & Chen, Z. P. A review of carbon dots produced from biomass wastes. Nanomaterials 10, 2316 (2020).
Lin, L., Luo, Y., Tsai, P., Wang, J. & Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC Trends Anal. Chem. 103, 87–101 (2018).
Sharma, A., Choi, H. K. & Lee, H. J. Carbon dots for the treatment of inflammatory diseases: An appraisal of in vitro and in vivo studies. Oxid. Med. Cell Longev. 25, 3076119 (2023).
Tran, T. V. et al. Effect of thermolysis condition on characteristics and nonsteroidal anti-inflammatory drugs (NSAIDs) absorbability of Fe-MIL-88B-derived mesoporous carbons. J. Environ. Chem. Eng. 7(5), 103356 (2019).
Karlicky, F., Ramanatha Datta, K. K., Otyepka, M. & Zboril, R. Halogenated graphenes: Rapidly growing family of graphene derivatives. ACS Nano 7, 6434–6464 (2013).
Jiang, K. et al. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 54(18), 5360–5363 (2015).
Shi, B. et al. Nitrogen and phosphorus co-doped carbon nanodots as a novel fluorescent probe for highly sensitive detection of Fe3+ in human serum and living cells. ACS Appl. Mater. Interfaces 8(17), 10717–10725 (2016).
Surendran, P. et al. Fluorescent carbon quantum dots from Ananas comosus waste peels: Promising material for NLO behavior, antibacterial, and antioxidant activities. Inorg. Chem. Commun. 124, 108397 (2021).
Nachimuthu, S. et al. Lawsonia inermis mediated synthesis of ZnO/Fe2O3 nanorods for photocatalysis–Biological treatment for the enhanced effluent treatment, antibacterial and antioxidant activities. Chem. Phys. Lett. 804, 139907 (2022).
Saro**i, P. et al. Design of V2O5 blocks decorated with garlic peel biochar nanoparticles: A sustainable catalyst for the degradation of methyl orange and its antioxidant activity. Materials 16(17), 5800 (2023).
Karthik, K., Dhanuskodi, S., Gobinath, C., Prabukumar, S. & Sivaramakrishnan, S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B Biol. 190, 8–20 (2019).
Karthik, K., Dhanuskodi, S., Gobinath, C., Prabukumar, S. & Sivaramakrishnan, S. Nanostructured CdO-NiO composite for multifunctional applications. J. Phys. Chem. Solids 112, 106–118 (2018).
Surendran, P. et al. Synthesis of fluorescent carbon quantum dots from Manihotesculenta waste peels for nonlinear optical and biological applications. Chem. Phys. Impact 8, 100515 (2024).
Chinnaiah, K. et al. Ag nanoparticles synthesized by Datura metel L. Leaf extract and their charge density distribution, electrochemical and biological performance. Chem. Phys. Lett. 807, 140083 (2022).
Karthik, K., Radhika, D., Gnanasangeetha, D., Sivarama Krishna, L. & Gurushankar, K. Y3+ and Sm3+ co-doped mixed metal oxide nanocomposite: Structural, electrochemical, photocatalytic, and antibacterial properties. Appl. Surf. Sci. Adv. 4, 100085 (2021).
Karthik, K. et al. Facile fabrication of novel ceria-based nanocomposite (CYO-CSO) via co-precipitation: Electrochemical, photocatalytic and antibacterial performances. J. Mol. Struct. 1256, 132519 (2022).
Ngoc, L. T. N., Moon, J. & Lee, Y. Antioxidants for improved skin appearance: Intracellular mechanism, challenges and future strategies. Int. J. Cosmet. Sci. 45, 299–314 (2023).
Innocenzi, P. & Stagi, L. Carbon dots as oxidant-antioxidant nanomaterials, understanding the structure-properties relationship. A critical review. Nano Today 50, 101837 (2023).
Chen, M. et al. Aggregation behavior and antioxidant properties of amphiphilic fullerene C60 derivatives cofunctionalized with cationic and nonionic hydrophilic groups. Langmuir 35, 6939–6949 (2019).
Zu, F. et al. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 184, 1899–1914 (2017).
Song, Y. et al. Investigation into the fluorescence quenching behaviors and applications of carbon dots. Nanoscale 6, 4676–4682 (2014).
Chen, M. et al. Magnetic and biocompatible fullerenol/Fe(III) microcapsules with antioxidant activities. ACS Appl. Bio. Mater. 3, 358–368 (2020).
Zhao, J. et al. Protective effects of Zingiberis Carbonisata-based carbon dots on diabetic liver injury in mice. J. Biomed. Nanotechnol. 18(8), 1975–1985 (2022).
Kong, H. et al. Carbon dots from Artemisiae Argyi Folium Carbonisata: Strengthening the antifrostbite ability. Arti Cells Nanomed. Biotechnol. 49(1), 11–19 (2021).
Moon, J. Y., Ngoc, L. T. N., Chae, M., Tran, V. V. & Lee, Y. C. Effects of microwave-assisted Opuntiahumi fusa extract in inhibiting the impacts of particulate matter on human keratinocyte skin cell. Antioxidants 9, 271 (2020).
Kong, B. et al. Carbon dots as nanocatalytic medicine for anti-inflammation therapy. J. Colloid Interface Sci. 611, 545–553 (2022).
Sadique, M. A. et al. Carbon Dots as a Potent Anti-inflammatory Agent. In Carbon Dots: Next-Generation Materials for Biomedical Applications (eds Singh, R. P. et al.) 21–224 (IOP Publishing, Bristol, 2022).
Wang, X. et al. Novel mulberry silkworm cocoon-derived carbon dots and their anti-inflammatory properties. Artif. Cells Nanomed. Biotechnol. 48(1), 68–76 (2020).
Zhang, M. et al. Multifunctional cerium doped carbon dots nanoplatform and its applications for wound healing. Chem. Eng. J. 423, 130301 (2021).
Li, Y. J. et al. Synthesis of self-assembled spermidine-carbon quantum dots effective against multidrug resistant bacteria. Adv. Healthcare Mater. 5, 2545–2554 (2016).
Jian, H. J. et al. Super- cationic carbon quantum dots synthesized from spermidine as an eye drop formulation for topical treatment of bacterial keratitis. ACS Nano 11, 6703–6716 (2017).
Liang, J. et al. Antibacterial activity and synergetic mechanism of carbon dots against gram positive and negative bacteria. ACS Appl. Bio Mater. 4(9), 6937–6945 (2021).
**ang, Y. et al. Rapid and superior bacteria killing of carbon quantum dots/ZnO decorated injectable folic acid-conjugated PDA hydrogel through dual-light triggered ROS and membrane permeability. Small 15(22), e1900322 (2019).
Boakye-Yiadom, K. O. et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 564, 308–317 (2019).
Hsu, C. Y., Chan, Y. P. & Chang, J. Antioxidant activity of extract from Polygonum cuspidatum. Biol. Res. 40(1), 13–21 (2007).
Bernfeld, P. Amylase α and β. Methods Enzymol. 1, 149–158 (1955).
Vane, J. R. & Botting, R. M. New insights into the mode of action of anti-inflammatory drugs. Inflamm. Res. 44(1), 1–10 (1995).
Acknowledgements
SK and RS sincerely acknowledge the receipt of a DST-AMT grant (No: DST/TDT/AM/2021/02) in support of this study. All the authors sincerely thank the Principal and the management of RVR and JC College of Engineering, Guntur, Andhra Pradesh, India for providing laboratory facilities and encouragement. The authors sincerely thank the Department of Microbiology, Katuri Medical College, Guntur, India, for supplying the clinical bacterial isolates, Dr. P. Krishna Kanthi, GITAM University, Visakhapatnam for technical support. In addition, the authors gratefully acknowledge the support from SAIF IIT Bombay, Advanced Research Laboratory and CNR Research centre, Avinashilingam Institute for Home Science and Higher Education For Women Coimbatore, and CoExAMMPC, Vignan University for the utilization of the characterization facility.
Author information
Authors and Affiliations
Contributions
S. K. designed the experiments and analyzed the experimental results; R. A. and P. D. performed the experimental work; S. K. and R. A. wrote the main manuscript text and drew the figures; R. S. performed the statistical analysis, prepared tables and assisted in manuscript preparation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kota, S., Dumpala, P., Sajja, R. et al. Heteroatom-doped carbon dots from medicinal plants as novel biomaterials for as-use biomedical applications in comparison with synthetic drug, zaltoprofen. Sci Rep 14, 13160 (2024). https://doi.org/10.1038/s41598-024-63700-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63700-w
- Springer Nature Limited