Abstract
The IVa subfamily of glycine-rich proteins (GRPs) comprises a group of glycine-rich RNA binding proteins referred to as GR-RBPa here. Previous studies have demonstrated functions of GR-RBPa proteins in regulating stress response in plants. However, the mechanisms responsible for the differential regulatory functions of GR-RBPa proteins in different plant species have not been fully elucidated. In this study, we identified and comprehensively studied a total of 34 GR-RBPa proteins from five plant species. Our analysis revealed that GR-RBPa proteins were further classified into two branches, with proteins in branch I being relatively more conserved than those in branch II. When subjected to identical stresses, these genes exhibited intensive and differential expression regulation in different plant species, corresponding to the enrichment of cis-acting regulatory elements involving in environmental and internal signaling in these genes. Unexpectedly, all GR-RBPa genes in branch I underwent intensive alternative splicing (AS) regulation, while almost all genes in branch II were only constitutively spliced, despite having more introns. This study highlights the complex and divergent regulations of a group of conserved RNA binding proteins in different plants when exposed to identical stress conditions. These species-specific regulations may have implications for stress responses and adaptations in different plant species.
Similar content being viewed by others
Introduction
Abiotic stresses, including soil salinity, drought, high and low temperatures, have emerged as major limiting factors affecting crop yield and quality1. With climate change intensifying and extreme weather events becoming more frequent, it is anticipated that abiotic stresses will increasingly impact crop production, posing a threat to global food security2,3. In response to these challenges, plants have evolved a diverse array of molecular mechanisms to rapidly perceive and adapt to environmental changes.
Eukaryotes respond to and adapt to environmental changes by employing transcriptional and post-transcriptional regulatory mechanisms4. Alternative splicing (AS) is a process wherein potential splicing sites on precursor mRNA (pre-mRNA) transcripts may or may not be utilized following transcription of the master gene, resulting in the generation of diverse mature mRNA isoforms from an intron-containing gene. AS regulation enhances the diversity of the transcriptome and proteome expressed from the same set of pre-mRNA transcripts5,6. Recent data from the human transcriptome and translatome have confirmed that AS can increase protein diversity7,8. Similarly, AS contributes to the diversity of the transcriptome and proteome in the plant kingdom9,10. Particularly, plants employ more intensive AS regulation to produce protein isoforms adapted to environmental stress11,12. Therefore, AS regulation represents an important post-transcriptional strategy in plants' response to environmental stress13,14. The application of AS regulation holds promise for enhancing the stress tolerance of crops in the face of global climate change10,15.
The majority of post-transcriptional RNA metabolism regulation is mediated by various RNA-binding proteins (RBPs)16. GR-RBP proteins represent a subgroup of RBPs within the fourth subfamily of the glycine-rich protein (GRP) superfamily. They are characterized by glycine-rich regions, (Gly)n-X (where n is typically an odd number and X can be any amino acid, including glycine), primarily located at the C-terminus of the protein17,18. Based on the motifs present in these proteins, GR-RBPs can be further categorized into four subclasses: IVa, IVb, IVc, and IVd. Among these, both IVa and IVd contain RNA recognition motifs (RRMs) at the N-terminus, with the distinction that IVa has one RRM, while IVd has two. Conversely, IVb and IVc have one or more CCHC zinc fingers located at the C-terminus, with IVb featuring an RRM at the N-terminus and IVc featuring a cold shock domain (CSD) at the N-terminus18,19. However, the classification of GR-RBP proteins remains unclear and occasionally contentious. For example, AT2G21660 (AtGRP7) was initially categorized as a protein in the IVa subgroup20, it was recently reclassified as a member of IVb21. Similarly, OsGRP4 and OsGRP5 in rice, exhibiting strong homology with AtGRP2/7 in Arabidopsis were initially considered GR-RBP proteins19,22. Additionally, GRMZM2G001850 was classified as a GR-RBP protein in maize54.
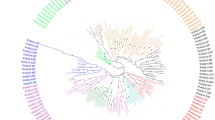
The GR-RBPa protein is chaeaterized by a conserved RNA Recognition Motif (RRM) domain located at the N-terminal and a glycine-rich C-terminal. Both terminals of GR-RBPa proteins play important roles in their functionality. Studies have shown that variations in the length of the glycine-rich region of AT3G223830 can lead to functional differences in cold stress adaptation, while another study suggested that the difference may originate from the N-terminal RRM domain itself55,56. Our analysis revealed significant variation in the length of the C-terminal region among different GR-RBPa members (Fig. 2). Moreover, GR-RBPa proteins from the five plant species could be further classified into two branches based on the conserved motifs they possess, consistent with previous findings24. For instance, motif 8 is exclusively found in branch I, while motifs 4, 7, and 9 are unique to branch II. GR-RBPa proteins within the same branch of a plant species are believed to exhibit more conservative molecular functions than those between different branches. For example, AT2G21660 and AT4G39260 of branch I regulate their own pre-mRNAs, cross-regulate each other’s pre-mRNAs, and share several downstream pre-mRNAs33,57. In contrast, although both AT4G13850 (belonging to branch II) and AT2G21660 have been shown to enhance cold resistance in plants, AT4G13850 promotes the germination of Arabidopsis seeds under salt and drought stresses, while AT2G21660 has a negative effect on seed germination and seedling growth under salt or dehydration stress conditions27,55,58,59. In addition, overexpression of AT3G23830 in branch II did not confer cold tolerance but only enhanced salt and drought resistance in the transgenic plants, similar to AT4G1385056. Interestingly, our protein–protein interaction analysis revealed that GR-RBPa proteins in branch I shared more interacting proteins than those in branch II, suggesting more conserved molecular function of GR-RBPa proteins in branch I (Fig. 3, Supplemental Fig. 2). Multiple and diverse roles of GR-RBPa proteins could be attributed to the different motifs they possess, as protein–protein interactions and binding of specific nucleotides of proteins may be affected by the motifs they contain60. Therefore, further systematic comparisons are needed in the future to confirm the functions of specific motifs of GR RBPa proteins.
GR-RBP proteins are known to play crucial roles in regulating plant growth and responding to stress17,31. Our analysis revealed that the promoters and introns of GR-RBPa genes are enriched with various cis-elements, including those involved in light signaling, hormone signaling, growth and development, and stress responses (Fig. 5). This indicates that GR-RBPa genes are subject to regulatory control at the expression level in response to various internal and external cues. Previous studies have shown that GR-RBP genes are highly induced by heat stress in sorghum61 and Pinellia ternate62. On the other hand, the expression level of LpGRP1 transcript in ryegrass was significantly induced with prolonged exposure to cold stress63. Additionally, the transcriptional levels of PpGRPs in Physcomitrella patents fluctuated during prolonged cold stress50. In our study, to ease and ensure the comparability between individual investigations, we use the same hydroponics-based experimental conditions when cultivate different plants. However, it is important to note that this approach may introduce extra stresses, such as oxygen and soil microbial deficiency, to the plants64. It's worth mentioning that we did not calculate whether there are differences and how significant they are in the gene transcription and splicing patterns of GR-RBP genes between plants cultivated in soils and those grown in water, as our focus was primarily on the effects generated from salt, drought and temperature here.
When we subjected the five plant species to salt, drought and temperature stresses, we observed significant and intricate changes in the expression of GR-RBPa genes compared to their expression levels in the original culture solution. For example, most GR-RBPa genes from maize and tomato showed transcriptional induction in response to all types of abiotic stress employed. Conversely, GR-RBPa genes in rice tended to be down-regulated under salt and cold stresses. Additionally, GR-RBPa genes in branch I were generally downregulated by all kinds of stress treatments, while those in branch II were more frequently induced by stress. The expression of some GR-RBPa genes exhibited fluctuations in response to different types of abiotic stress or even different durations of a particular stress. Moreover, homologous GR-RBPa genes from the same plant species could be regulated in opposite directions by the same stress. However, transcriptional regulations of GR-RBPa genes did not exhibit clear divergence between monocots and dicots. In conclusion, transcriptional levels of these GR-RBPa genes are not conserved among the plant species we tested. Therefore, it is essential to investigate in future studies whether transcriptional regulation of the GR-RBPa genes is controlled by coordinated modulations between different cis-elements. Alternatively, there could be other unidentified cis-elements that significantly affect transcriptional levels.
Accumulating studies have demonstrated that pre-mRNA alternative splicing (AS) regulation plays essential roles in plant growth and stress response because alterations in the relative abundances of different transcript isoforms can affect the abundance and diversity of protein products65,66,67,68. In our study, the results of gene structure analysis showed a clear distinction in the number of introns between GR-RBPa genes in branch I and branch II. The genes in branch I have fewer introns (only 1 or 2) than those in branch II (Fig. 4), suggesting a potential co-evolution of gene structure and protein function among GR-RBPa proteins in plants. Surprisingly, RT-PCR results demonstrated that all of the GR-RBPa genes in branch I tested were found to be alternatively spliced and subsequently generated multiple mRNA isoforms, while nearly all GR-RBPa genes in branch II were only constitutively spliced, even though they contained more introns (Fig. 9). In addition, AS patterns changed significantly among GR-RBPa genes in branch I, for various types of stress or different durations of the same stress treatment could induce different AS variations in pre-mRNAs of these genes. Moreover, AS regulations of homologous GR-RBPa genes changed significantly among different plant species, and no clear divergence of AS regulation model was found between monocot and dicot species. These findings highlight the complexity and variability of AS regulations in coding genes of the RNA binding proteins (RBPs), which have also been recently reported in another family, serine/arginine-rich (SR) protein45,69.
The reason for the differential response of different branches of GR-RBPa genes to identical stress at both transcription and splicing levels remains unclear. It remains uncertain whether or not the evolution in gene structure results in specific regulation of the master genes themselves, and subsequently affecting downstream genes at the genome-wide level. Studies in different plants have shown that AS of pre-mRNA of GR-RBPa proteins can alter the splicing patterns of downstream genes57,70. In our experiments, there were also significant changes in the abundances of different AS transcripts of GR-RBPa proteins when the plants were subjected to environmental stresses. In-silico analysis has also indicated that potential truncated proteins may be generated through AS of AT2g21660 gene (Fig. 9c–f). Existing evidence supports that AS-induced protein variants have different functions in response to environmental changes. For instance, HAB1 protein variants generated through pre-mRNA AS regulation play opposite roles in ABA signaling71,72. Additionally, two protein variants of OsZIP1 generated through pre-mRNA AS regulate development of rice plant under light and dark conditions, respectively73. However, further functional studies are required to confirm how different GR-RBPa alternatively spliced variants affect the stress response and adaptation in plants.
In conclusion, the intricate and diverse expression regulation of GR-RBPa genes across different plant species, at both transcriptional and pre-mRNA splicing levels, prompts questions about the contribution of these regulatory mechanisms to plant development and stress adaptation. Despite sharing relatively conserved functional domains, the impact of their diverse expression patterns on growth and development of the plants remains unclear. Understanding the biological significance of the variations in each pre-mRNA isoform could be crucial for future application of this gene family in breeding crops for enhanced stress tolerance.
Data availability
All main data of the study appear in the submitted article. Supplementary data are available online.
References
Kopecka, R., Kameniarova, M., Cerny, M., Brzobohaty, B. & Novak, J. Abiotic stress in crop production. Int. J. Mol. Sci. 24(7), 6603 (2023).
Batley, J. & Edwards, D. The application of genomics and bioinformatics to accelerate crop improvement in a changing climate. Curr. Opin. Plant Biol. 30, 78–81 (2016).
Suprasanna, P. Plant abiotic stress tolerance: Insights into resilience build-up. J. Biosci. 45(1), 120 (2020).
Reddy, A. S., Marquez, Y., Kalyna, M. & Barta, A. Complexity of the alternative splicing landscape in plants. Plant Cell 25(10), 3657–3683 (2013).
Filichkin, S. A. et al. Abiotic stresses modulate landscape of poplar transcriptome via alternative splicing, differential intron retention, and isoform ratio switching. Front. Plant Sci. 9, 5 (2018).
Filichkin, S., Priest, H. D., Megraw, M. & Mockler, T. C. Alternative splicing in plants: Directing traffic at the crossroads of adaptation and environmental stress. Curr. Opin. Plant Biol. 24, 125–135 (2015).
Floor, S. N. & Doudna, J. A. Tunable protein synthesis by transcript isoforms in human cells. Elife. 5, e10921 (2016).
Liu, Y. et al. Impact of alternative splicing on the human proteome. Cell Rep. 20(5), 1229–1241 (2017).
Yu, H., Tian, C., Yu, Y. & Jiao, Y. Transcriptome survey of the contribution of alternative splicing to proteome diversity in Arabidopsis thaliana. Mol. Plant 9(5), 749–752 (2016).
Gao, B., Chen, M. & Oliver, M. J. Alternative splicing: From abiotic stress tolerance to evolutionary genomics. Int. J. Mol. Sci. 24(7), 6708 (2023).
Chaudhary, S., Jabre, I., Reddy, A. S. N., Staiger, D. & Syed, N. H. Perspective on alternative splicing and proteome complexity in plants. Trends Plant Sci. 24(6), 496–506 (2019).
Palusa, S. G. & Reddy, A. S. N. Differential recruitment of splice variants from SR pre-mRNAs to polysomes during development and in response to stresses. Plant Cell Physiol. 56(3), 421–427 (2015).
Laloum, T., Martin, G. & Duque, P. Alternative splicing control of abiotic stress responses. Trends Plant Sci. 23(2), 140–150 (2018).
Zhu, D. et al. The features and regulation of co-transcriptional splicing in Arabidopsis. Mol. Plant 13(2), 278–294 (2020).
Shang, X., Cao, Y. & Ma, L. Alternative splicing in plant genes: A means of regulating the environmental fitness of plants. Int. J. Mol. Sci. 18(2), 432 (2017).
Muthusamy, M., Kim, J. H., Kim, J. A. & Lee, S. I. Plant RNA binding proteins as critical modulators in drought, high salinity, heat, and cold stress responses: An updated overview. Int. J. Mol. Sci. 22(13), 6731 (2021).
Czolpinska, M. & Rurek, M. Plant glycine-rich proteins in stress response: An emerging, still prospective story. Front. Plant Sci. 9, 302 (2018).
Sachetto-Martins, G., Franco, L.O., Oliveira, D.E. Plant glycine-rich proteins: A family or just proteins with a common motif? Biochimica et biophysica acta. 1492(1), 1–14 (2000).
Ma, L., Cheng, K., Li, J., Deng, Z., Zhang, C. & Zhu, H. Roles of plant glycine-rich RNA-binding proteins in development and stress responses. Int. J. Mol. Sci. 22(11), 5849 (2021).
Mangeon, A., Junqueira, R. M. & Sachetto-Martins, G. Functional diversity of the plant glycine-rich proteins superfamily. Plant Signal Behav. 5(2), 99–104 (2010).
Lu, Y. et al. Genome-wide identification and expression analysis of glycine-rich RNA-binding protein family in sweet potato wild relative Ipomoea trifida. Gene 686, 177–186 (2019).
Kim, J. Y. et al. Glycine-rich RNA-binding proteins are functionally conserved in Arabidopsis thaliana and Oryza sativa during cold adaptation process. J. Exp. Bot. 61(9), 2317–2325 (2010).
Zhang, J., Zhao, Y., **ao, H., Zheng, Y. & Yue, B. Genome-wide identification, evolution, and expression analysis of RNA-binding glycine-rich protein family in maize. J. Integr. Plant Biol. 56(10), 1020–1031 (2014).
Krishnamurthy, P., Kim, J. A., Jeong, M. J., Kang, C. H. & Lee, S. I. Defining the RNA-binding glycine-rich (RBG) gene superfamily: New insights into nomenclature, phylogeny, and evolutionary trends obtained by genome-wide comparative analysis of Arabidopsis, Chinese cabbage, rice and maize genomes. Mol. Genet. Genom. 290(6), 2279–2295 (2015).
Lorkovic, Z. J. Role of plant RNA-binding proteins in development, stress response and genome organization. Trends Plant Sci. 14(4), 229–236 (2009).
Lorković, Z. J. & Barta, A. Genome analysis: RNA recognition motif (RRM) and K homology (KH) domain RNA-binding proteins from the flowering plant Arabidopsis thaliana. Nucleic Acids Res. 30(3), 623–635 (2002).
Kim, J. S. et al. Glycine-rich RNA-binding protein 7 affects abiotic stress responses by regulating stomata opening and closing in Arabidopsis thaliana. Plant J. 55(3), 455–466 (2008).
Yang, D. H. et al. Expression of Arabidopsis glycine-rich RNA-binding protein AtGRP2 or AtGRP7 improves grain yield of rice (Oryza sativa) under drought stress conditions. Plant Sci. 214, 106–112 (2014).
Sahi, C., Agarwal, M., Singh, A. & Grover, A. Molecular characterization of a novel isoform of rice (Oryza sativa L.) glycine rich-RNA binding protein and evidence for its involvement in high temperature stress response. Plant Sci. 173(2), 144–155 (2007).
Xu, W. et al. OsGRP3 enhances drought resistance by altering phenylpropanoid biosynthesis pathway in rice (Oryza sativa L.). Int. J. Mol. Sci. 23(13), 7045 (2022).
Ma, L. et al. SlRBP1 promotes translational efficiency via SleIF4A2 to maintain chloroplast function in tomato. Plant Cell 34(7), 2747–2764 (2022).
Heintzen, C., Nater, M., Apel, K. & Staiger, D. AtGRP7, a nuclear RNA-binding protein as a component of a circadian-regulated negative feedback loop in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 94(16), 8515–8520 (1997).
Staiger, D., Zecca, L., Kirk, D. A. W., Apel, K. & Eckstein, L. The circadian clock regulated RNA-binding protein AtGRP7 autoregulates its expression by influencing alternative splicing of its own pre-mRNA. Plant J. Cell Mol. Biol. 33(2), 361–371 (2003).
Mangeon, A. et al. AtGRP5, a vacuole-located glycine-rich protein involved in cell elongation. Planta 230(2), 253–265 (2009).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38(7), 3022–3027 (2021).
Gasteiger, E. et al. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31(13), 3784–3788 (2003).
Hu, B. et al. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 31(8), 1296–1297 (2015).
Szklarczyk, D. et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47(D1), D607–D613 (2019).
Rombauts, S., Déhais, P., Montagu, M.V., Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 27(1), 295–296 (1999).
Magali, L. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30(1), 325–327 (2002).
Maruyama, K. et al. Design of an optimal promoter involved in the heat-induced transcriptional pathway in Arabidopsis, soybean, rice and maize. Plant J. 89(4), 671–680 (2017).
Hu, J., Israeli, A., Ori, N. & Sun, T. P. The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell 30(8), 1710–1728 (2018).
**, Y., Liu, F., Huang, W., Sun, Q. & Huang, X. Identification of reliable reference genes for qRT-PCR in the ephemeral plant Arabidopsis pumila based on full-length transcriptome data. Sci. Rep. 9(1), 8408 (2019).
Lin, Y. et al. Validation of potential reference genes for qPCR in maize across abiotic stresses, hormone treatments, and tissue types. PLoS One 9(5), e95445 (2014).
Chen, S., Mo, Y., Zhang, Y., Zhu, H. & Ling, Y. Insights into sweet potato SR proteins: From evolution to species-specific expression and alternative splicing. Planta 256(4), 72 (2022).
Li-Wei, Z. et al. Soluble oligosaccharide and small heat shock protein correlated with seed germination and vigor during hybrid rice seed maturation. Acta Agronomica Sinica 42(05), 714–724 (2016).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protocols 3(6), 1101–1108 (2008).
Condit, C. M. & Meagher, R. B. Expression of a gene encoding a glycine-rich protein in petunia. Mol. Cell. Biol. 7(12), 4273–4279 (1987).
Steven, N. & Richard, D. V. Two cDNAs from Arabidopsis thaliana encode putative RNA binding proteins containing glycine-rich domains. Plant Mol. Biol. 21(4), 695–699 (1993).
Tsuyoshi, N., Yukihiro, K. & Naoki, S. Cloning and characterization of glycine-rich RNA-binding protein cDNAs in the moss Physcomitrella patens. Plant Cell Physiol. 45(1), 48–56 (2004).
Kim, J. Y. et al. Zinc finger-containing glycine-rich RNA-binding protein in Oryza sativa has an RNA chaperone activity under cold stress conditions. Plant Cell Environ. 33(5), 759–768 (2010).
Kim, M. K., Jung, H. J., Kim, D. H. & Kang, H. Characterization of glycine-rich RNA-binding proteins inBrassica napusunder stress conditions. Physiol. Plantarum 146(3), 297–307 (2012).
Lu, X., Cheng, Y., Gao, M., Li, M. & Xu, X. Molecular characterization, expression pattern and function analysis of glycine-rich protein genes under stresses in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Front. Genet. 11, 774 (2020).
Wang, P. et al. Factors influencing gene family size variation among related species in a plant family, Solanaceae. Genome Biol. Evol. 10(10), 2596–2613 (2018).
Kim, J. S. et al. Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote the cold adaptation process in Escherichia coli. Nucleic Acids Res. 35(2), 506–516 (2007).
Kwak, K. J., Kim, Y. O. & Kang, H. Characterization of transgenic Arabidopsis plants overexpressing GR-RBP4 under high salinity, dehydration, or cold stress. J. Exp. Bot. 56(421), 3007–3016 (2005).
Streitner, C. et al. An hnRNP-like RNA-binding protein affects alternative splicing by in vivo interaction with transcripts in Arabidopsis thaliana. Nucleic Acids Res. 40(22), 11240–11255 (2012).
Cao, S., Jiang, L., Song, S., **g, R. & Xu, G. AtGRP7 is involved in the regulation of abscisic acid and stress responses in arabidopsis. Cell. Mol. Biol. Lett. 11(4), 526–535 (2006).
Ciuzan, O., Lazar, S. L., Lung, M. L., Pop, O. L. & Pamfil, D. Involvement of the glycine-rich RNA-binding proteins (GRP) in plant abiotic stress response: A comparison between GRP 2 and GRP 7. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Horticult. 72(1), 61–67 (2015).
Cruz-Gallardo, I. et al. LARP4A recognizes polyA RNA via a novel binding mechanism mediated by disordered regions and involving the PAM2w motif, revealing interplay between PABP, LARP4A and mRNA. Nucleic Acids Res. 47(8), 4272–4291 (2019).
Singh, S. et al. A temperature-responsive gene in sorghum encodes a glycine-rich protein that interacts with calmodulin. Biochimie 137, 115–123 (2017).
Zhu, Y. et al. A comparative proteomic analysis of Pinellia ternata leaves exposed to heat stress. Int. J. Mol. Sci. 14(10), 20614–20634 (2013).
Shinozuka, H. et al. Gene expression and genetic map** analyses of a perennial ryegrass glycine-rich RNA-binding protein gene suggest a role in cold adaptation. Mol. Genet. Genom. 275(4), 399–408 (2006).
Blok, C., Jackson, B. E., Guo, X., de Visser, P. H. B. & Marcelis, L. F. M. Maximum plant uptakes for water, nutrients, and oxygen are not always met by irrigation rate and distribution in water-based cultivation systems. Front. Plant Sci. 8, 562 (2017).
Ling, Y., Mahfouz, M. M. & Zhou, S. Pre-mRNA alternative splicing as a modulator for heat stress response in plants. Trends Plant Sci. 26(11), 1153–1170 (2021).
Long, W., Fan, X. & Feng, Y. Two environmental signal-driven RNA metabolic processes: Alternative splicing and translation. Plant Cell Environ. 46(3), 718–732 (2023).
Zhen, W. et al. Modulation of plant development and chilling stress responses by alternative splicing events under control of the spliceosome protein SmEb in Arabidopsis. Plant Cell Environ. 45(9), 2762–2779 (2022).
Haroon, B. et al. The rice serine/arginine splicing factor RS33 regulates pre-mRNA splicing during abiotic stress responses. Cells. 11(11), 1796 (2022).
Chen, S. et al. Transcription and splicing variations of SR genes accompany with genome-wide accumulation of long-introns in pine. Plant Sci. 342, 112056 (2024).
Chen, Q. et al. Genome-wide association analyses reveal the importance of alternative splicing in diversifying gene function and regulating phenotypic variation in maize. Plant Cell 30(7), 1404–1423 (2018).
Wang, Z. et al. ABA signalling is fine-tuned by antagonistic HAB1 variants. Nat. Commun. 6, 8138 (2015).
Zhan, X. et al. An Arabidopsis PWI and RRM motif-containing protein is critical for pre-mRNA splicing and ABA responses. Nat. Commun. 6, 8139 (2015).
Bhatnagar, A. et al. Two splice forms of OsbZIP1, a homolog of AtHY5, function to regulate skotomorphogenesis and photomorphogenesis in rice. Plant Physiol. 193(1), 426–447 (2023).
Funding
This study was funded by Guangdong Basic and Applied Basic Research Foundation (2024A1515012394), Innovative team for development and utilization of germplasm resource of saline-alkali tolerant plants (headed by Prof. **ngyu Jiang), and the Special Project of Seed Industry Vitalization under Rural Revitalization Strategy in Guangdong Province (2022NPY00014).
Author information
Authors and Affiliations
Contributions
LY had the idea, designed the project and revised the manuscript with assistance from JX; ZYJ and MY ran out most the experiments and wrote the draft of manuscript with assistance from LJ, LL, RL and GY; ZYQ, HY and ZH provided the plant materials and gave suggestions in revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Mo, Y., Li, J. et al. Divergence in regulatory mechanisms of GR-RBP genes in different plants under abiotic stress. Sci Rep 14, 8743 (2024). https://doi.org/10.1038/s41598-024-59341-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59341-8
- Springer Nature Limited