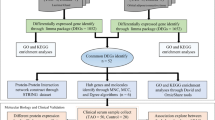
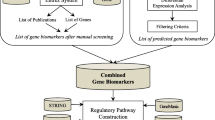
Abstract
Thyroid eye disease (TED), an autoimmune inflammatory disorder affecting the orbit, exhibits a range of clinical manifestations. While the disease presentation can vary, cases that adhere to a prototypical pattern typically commence with mild symptoms that subsequently escalate in severity before entering a phase of stabilization. Notably, the metabolic activity of cells implicated in the disease substantially deviates from that of healthy cells, with purine metabolism representing a critical facet of cellular material metabolism by supplying components essential for DNA and RNA synthesis. Nevertheless, the precise involvement of Purine Metabolism Genes (PMGs) in the defensive mechanism against TED remains largely unexplored. The present study employed a bioinformatics approach to identify and validate potential PMGs associated with TED. A curated set of 65 candidate PMGs was utilized to uncover novel PMGs through a combination of differential expression analysis and a PMG dataset. Furthermore, GSEA and GSVA were employed to explore the biological functions and pathways associated with the newly identified PMGs. Subsequently, the Lasso regression and SVM-RFE algorithms were applied to identify hub genes and assess the diagnostic efficacy of the top 10 PMGs in distinguishing TED. Additionally, the relationship between hub PMGs and clinical characteristics was investigated. Finally, the expression levels of the identified ten PMGs were validated using the GSE58331 and GSE105149 datasets. This study revealed ten PMGs related with TED. PRPS2, PFAS, ATIC, NT5C1A, POLR2E, POLR2F, POLR3B, PDE3A, ADSS, and NTPCR are among the PMGs. The biological function investigation revealed their participation in processes such as RNA splicing, purine-containing chemical metabolism, and purine nucleotide metabolism. Furthermore, the diagnostic performance of the 10 PMGs in differentiating TED was encouraging. This study was effective in identifying ten PMGs linked to TED. These findings provide light on potential new biomarkers for TED and open up possibilities for tracking disease development.
Similar content being viewed by others
Introduction
Thyroid eye disease (TED), commonly known as Graves' ophthalmopathy or Graves' orbitopathy, is an inflammatory condition characterized by ocular tissue involvement. This disease is distinguished by lymphocyte infiltration, an increase in orbital fat, and edema of the extraocular muscles1,2. TED is frequently connected with Graves' disease, a systemic autoimmune ailment marked by endocrine symptoms that have a major impact on afflicted persons' quality of life3. TED is dangerous since it can cause visual impairment, functional incapacity, and deformity. While TED is most commonly seen in people with Graves' hyperthyroidism, it can also appear in hypothyroid or euthyroid patients4. TED usually appears 18 months after the beginning of endocrine symptoms, with roughly 80% of patients having contemporaneous signs. While TED and Graves' hyperthyroidism can affect people of various ages, it is more common in women between the ages of 30 and 50. TED is expected to affect around 16 cases per 100,000 women and three cases per 100,000 men per year5,6. Cigarette smoking, a lengthy history of Graves' hyperthyroidism, inadequate thyroid dysfunction care, and past radioactive iodine therapy have all been recognized as risk factors7. TED is frequently diagnosed by a thorough medical history and physical examination. Ophthalmic symptoms are reported in up to 50% of Graves' hyperthyroidism patients8. Therefore, understanding the underlying molecular mechanisms implicated in TED is critical for develo** innovative treatment options that might successfully prevent disease recurrence and improve patient outcomes.
Nutrient intake and metabolism are critical processes for all living creatures' existence. Metabolic reprogramming is important in tumor cell growth and survival in the context of cancer. Recent research has shown that oncogenic transition causes a different metabolic profile in tumor cells, resulting in changes in the tumor microenvironment (TME). The TME is a complex environment composed of several cell types buried inside a complicated matrix, characterized by insufficient oxygen and nutrition availability due to damaged or poorly established vasculature9. The study of non-tumor immune infiltration is becoming increasingly important as research advances. The immune response is inextricably connected to substantial changes in tissue metabolism, such as nutritional depletion, increased oxygen demand, and the production of reactive nitrogen and oxygen species36. Such spatial distribution of PFAS within the brain imposes a consequential toxicological burden on the central nervous system, reinforcing the imperative for further rigorous examination. Collectively, these findings serve as an edifying foundation for understanding the ingress and bioaccumulation patterns of PFAS, thereby delineating avenues for subsequent investigative scrutiny and potential therapeutic intervention strategies37.
The findings of Antonacopoulou's study shed light on the higher expression of POLR2F and PRNP in carcinomas compared to normal tissue samples, indicating a possible role in colorectal cancer38. Lin established a 6-RBP gene signature consisting of POLR2F, DYNC1H1, SMAD9, TRIM21, BRCA1, and ERI1 in another bioinformatics work, allowing for a complete assessment of glioma and ischemic stroke. The RBPS 6-RBP gene signature, in particular, revealed independent prognostic potential for overall survival. Surprisingly, the study discovered a relationship between SMAD9 overexpression and dementia, indicating a possible link between SMAD9 and cognitive abnormalities. Conversely, POLR2F downregulation appears to be linked to age-related hypoxic stress, highlighting its possible role in physiological responses to oxygen deprivation during the aging process39. In the specialized milieu of TED, PMGs such as PFAS and POLR2F have been intriguingly implicated, as discerned from the patient cohort under our investigative lens, thereby lending heightened credence and legitimacy to our findings. Corroborating with the insights gleaned from the GSE105149 study, which suggested that a Purine Metabolism-related signature could effectively serve as a prognostic predictor, our results intertwine with this existing knowledge matrix, though it is pivotal to underscore the fact that the specific gene alterations associated with Purine Metabolism have hitherto been relatively underexplored in the scientific literature. In summation, our investigations not only substantively augment the nascent corpus of knowledge within this specific domain but also bequeath crucial insights, delicately poised to catalyze forthcoming investigational ventures and therapeutic innovations. By doing so, we carve a pathway towards a more nuanced understanding and potential novel therapeutic interventions in the complex and intricate realm of TED.
The TME is a complex environment that includes not only malignant cells but also a wide range of non-malignant cellular elements, vascular networks, and extracellular matrix. Myriad immune cell subtypes play critical regulatory roles in this dynamic ecology. Cumulative data highlights the substantial impact of the complicated interplay between neoplastic cells and the many aspects of the TME, which frequently results in immunological evasion of the immunological illnesses, stimulating Immune disease proliferation, recurrence, and metastasis. Despite great advances in cancer immunotherapy, the treatment paradigm is riddled with difficulties that prevent its widespread effectiveness. As a result, discovering new treatment targets and predicting biomarkers is critical for improving and enhancing immunotherapeutic efficacies. In this setting, an in-depth examination of immune cell infiltration inside the TME is critical. A slew of clinical studies have been conducted in the last 6–8 years to investigate various immune modulators, owing to a better knowledge of the molecular pathways underlying the pathogenesis of TED40. It is commonly assumed that thyroid autoantibodies cause TED by eliciting a cross-reactive reaction against the thyrotropin receptor (TSHR) and the IGF-1R on ocular fibroblasts. This sets off a chain reaction of events involving B cell-mediated mechanisms, such as autoantigen detection on orbital fibroblasts, subsequent T cell activation, migration of T cells, macrophages, and mast cells to the orbit, and an increasing release of proinflammatory cytokines41,42. Consequently, orbital fibroblasts become activated, initiating a self-perpetuating cycle characterized by increased cytokine production, proliferation of myofibroblasts and adipocytes, and excessive secretion of hyaluronic acid. Targeted therapeutic strategies based on this intricate pathophysiological model would naturally involve interventions aimed at preventing T cell activation and depletion43.
Agents targeting CD3, including ciclosporin, otelixizumab, and teplizumab, have demonstrated significant potential in attenuating T cell activity. Additionally, rituximab treatment offers an adjunctive therapeutic approach for B cell depletion44. Notably, the inhibition of cytokines, exemplified by tocilizumab, and the utilization of anti-TNF alpha monoclonal antibodies have been investigated45. Moreover, the comprehensive suppression of immune cell proliferation through the use of antimetabolites such as azathioprine and mycophenolate has shown promising outcomes in studies of TED therapy46. Expanding upon previous investigations, we further explored the expression of PMGs within the immune microenvironment. T cells, follicular helper cells, and neutrophils were found to exhibit upregulated expression in the treatment group. These findings provide additional evidence for the involvement of PMGs in the pathogenesis of TED, particularly with respect to inflammation and immune response.
The quest to identify biomarkers and their interrelationships with TED remains an understudied domain in extant scientific literature. Contemporary research endeavors, employing bioinformatic analyses, have begun to elucidate the metabolic correlates of ocular maladies47,48,49. For instance, studies by Liu et al. deployed Weighted Gene Coexpression Network Analysis to pinpoint hub genes integral to TED. Concurrently, Hu et al. innovatively fashioned a bioinformatic model to identify a cadre of 11 salient genes implicated in thyroid eye disease, including ATP6V1A and PTGES3 among others. In a related vein, Huang et al. discerned six significant genes for diabetic retinopathy through an amalgamation of comprehensive bioinformatics scrutiny and in vivo validation, including CD44 and CDC42, to name a few. Remarkably, the nexus between Purine Metabolism and TED remains a terra incognita in the research landscape.
In the burgeoning frontier of cancer immunotherapy, this investigation meticulously delineates a cardinal function for PMGs, engendering empirical correlations with pivotal immunological indicators and thereby, enriching our apprehension of its immunomodulatory interactions. The findings not only amplify the prevailing paradigm of PMGs' involvement in the intricate interplay between TED and host immunity but also carve out a novel scientific trajectory, intertwining detailed molecular insights with clinical relevance. Furthermore, this work forges ahead, providing clinicians with invaluable insights, potentially sculpting the future framework for a more nuanced understanding and therapeutic strategy toward TED, thereby fostering an enriched clinical insight that is anticipated to steer future exploratory and therapeutic endeavours in the entwined realms of immunotherapy and ocular pathophysiology. However, it is critical to recognize the constraints on our study. First, the integrity of our verified PMGs prognostic signature is inextricably linked to GEO datasets, emphasizing the importance of independent validation via external data repositories—resources that have proven difficult in the current context. Second, while our bioinformatics analysis provides important early insights into the functional dynamics of PMGs in oncological contexts, these computational discoveries are simply the starting point for a more detailed investigation. There is still an urgent need for empirical confirmation via rigorous in vitro and in vivo experimental designs to transform these preliminary findings into effective treatment solutions. Finally, the post-translational landscape—important in altering intracellular signaling cascades and regulatory molecule functional activities—represents a gap in our current understanding of PMGs. Current databases provide insufficient information on these crucial biochemical alterations, indicating a need for more research.
Conclusions
The etiology and pathogenesis of TED are orchestrated by a complex interplay of numerous molecular targets, cellular pathways, signal transduction mechanisms, and regulatory networks, which manifest as both synergistic and bidirectional modulatory effects. Within the cohort of PMGs examined, pivotal regulators, notably PRPS2, PFAS, ATIC, NT5C1A, POLR2E, POLR2F, POLR3B, PDE3A, ADSS, and NTPCR, have been delineated. Singular emphasis has been placed on PFAS and POLR2F for their salient roles in disease dynamics. This investigation augments our nuanced understanding of the intricate interrelations between PMGs and TED, thereby charting a course for future innovations in diagnostic modalities and therapeutic interventions for this multifaceted disorder.
Data availability
The datasets generated during and/or analyzed during the current study are available in the appendix.
Abbreviations
- TED:
-
Thyroid eye disease
- GO:
-
Gene ontology
- TCM:
-
Traditional Chinese medicine
- MF:
-
Molecular functions
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- GEO:
-
Gene expression omnibus
- PMGs:
-
Purine metabolism genes
- BP:
-
Biological processes
- CC:
-
Cellular components
- DEGs:
-
Differentially expressed genes
References
Belliveau, M. J. & Jordan, D. R. Thyroid eye disease. CMAJ 185(9), 797. https://doi.org/10.1503/cmaj.121815 (2013).
Ugradar, S. et al. Teprotumumab for the treatment of chronic thyroid eye disease. Eye (Lond) 36(8), 1553–1559. https://doi.org/10.1038/s41433-021-01593-z (2022).
Girnita, L., Smith, T. J. & Janssen, J. It takes two to Tango: IGF-I and TSH receptors in thyroid eye disease. J. Clin. Endocrinol. Metab. 107, S1–S12. https://doi.org/10.1210/clinem/dgac045 (2022).
Weiler, D. L. Thyroid eye disease: a review. Clin. Exp. Optom. 100(1), 20–25. https://doi.org/10.1111/cxo.12472 (2017).
Douglas, R. S. et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl. J. Med. 382(4), 341–352. https://doi.org/10.1056/NEJMoa1910434 (2020).
Nie, T. & Lamb, Y. N. Teprotumumab: a review in thyroid eye disease. DRUGS 82(17), 1663–1670. https://doi.org/10.1007/s40265-022-01804-1 (2022).
Jain, A. P., Jaru-Ampornpan, P. & Douglas, R. S. Thyroid eye disease: redefining its management-A review. Clin. Exp. Ophthalmol. 49(2), 203–211. https://doi.org/10.1111/ceo.13899 (2021).
Men, C. J., Kossler, A. L. & Wester, S. T. Updates on the understanding and management of thyroid eye disease. Ther. Adv. Ophthalmol. 13, 970386640. https://doi.org/10.1177/25158414211027760 (2021).
Jo, D. H., Kim, J. H. & Kim, J. H. Tumor environment of retinoblastoma intraocular cancer. Adv. Exp. Med. Biol. 1296, 349–358. https://doi.org/10.1007/978-3-030-59038-3_21 (2020).
**a, L. et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 20(1), 28. https://doi.org/10.1186/s12943-021-01316-8 (2021).
Zhang, Y. & Zhang, Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 17(8), 807–821. https://doi.org/10.1038/s41423-020-0488-6 (2020).
Yang, S. et al. A novel purine and uric metabolism signature predicting the prognosis of hepatocellular carcinoma. Front. Genet. 13, 942267. https://doi.org/10.3389/fgene.2022.942267 (2022).
Liu, J. et al. Targeting purine metabolism in ovarian cancer. J. Ovarian Res. 15(1), 93. https://doi.org/10.1186/s13048-022-01022-z (2022).
Yin, J. et al. Potential mechanisms connecting purine metabolism and cancer therapy. Front. Immunol. 9, 1697. https://doi.org/10.3389/fimmu.2018.01697 (2018).
Shatova, O. P. et al. Metformin impact on purine metabolism in breast cancer. Biomed. Khim. 62(3), 302–305 (2016).
Chen, X. & Chen, J. miR-10b-5p-mediated upregulation of PIEZO1 predicts poor prognosis and links to purine metabolism in breast cancer. Genomics 114(3), 110351. https://doi.org/10.18097/PBMC20166203302 (2022).
Ebeling, P. R. et al. Secondary osteoporosis. Endocr. Rev. 43(2), 240–313. https://doi.org/10.1210/endrev/bnab028 (2022).
Zhao, S. T. et al. Visualization analysis of the international standard ISO/TC 249 for traditional Chinese medicine. Dig. Chinese Med. 5(2), 103–111 (2022).
Wu, Z. et al. A novel Alzheimer’s disease prognostic signature: identification and analysis of glutamine metabolism genes in immunogenicity and immunotherapy efficacy. Sci. Rep. 13(1), 6895. https://doi.org/10.1038/s41598-023-33277-x (2023).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12(5), 453–457. https://doi.org/10.1038/nmeth.3337 (2015).
Zhang, Y., Topham, D. J., Thakar, J. & Qiu, X. FUNNEL-GSEA: FUNctioNal ELastic-net regression in time-course gene set enrichment analysis. Bioinformatics 33(13), 1944–1952. https://doi.org/10.1093/bioinformatics/btx104 (2017).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 14, 7. https://doi.org/10.1186/1471-2105-14-7 (2013).
Hoang, T. D., Stocker, D. J., Chou, E. L. & Burch, H. B. 2022 update on clinical management of Graves disease and thyroid eye disease. Endocrinol. Metab. Clin. North Am. 51(2), 287–304. https://doi.org/10.1016/j.ecl.2021.12.004 (2022).
Barbesino, G., Salvi, M. & Freitag, S. K. Future projections in thyroid eye disease. J. Clin. Endocrinol. Metab. 107, S47–S56. https://doi.org/10.1210/clinem/dgac252 (2022).
Khong, J. J. & McNab, A. Medical treatment in thyroid eye disease in 2020. Br J. Ophthalmol. 105(3), 299–305. https://doi.org/10.1136/bjophthalmol-2020-316051 (2021).
Lee, M. H. et al. Risk factors of thyroid eye disease. Endocr. Pract. 27(3), 245–253. https://doi.org/10.1016/j.eprac.2020.11.011 (2021).
Rashad, R., Pinto, R., Li, E., Sohrab, M. & Distefano, A. G. Thyroid eye disease. Life (Basel) https://doi.org/10.3390/life12122084 (2022).
Mukherjee, S. et al. Familiarity breeds strategy in silico untangling of the molecular complexity on course of autoimmune liver disease-to-hepatocellular carcinoma transition predicts novel transcriptional signatures. Cells-Basel 10, 1917 (2021).
Yang, K., Li, J. & Tao, L. Purine metabolism in the development of osteoporosis. Biomed. Pharmacother. 155, 113784. https://doi.org/10.1016/j.biopha.2022.113784 (2022).
Furuhashi, M. New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. Am J Physiol Endocrinol Metab 319(5), E827–E834. https://doi.org/10.1152/ajpendo.00378.2020 (2020).
Yu, F. et al. Breast cancer prognosis signature: linking risk stratification to disease subtypes. Brief. Bioinform. 20(6), 2130–2140. https://doi.org/10.1093/bib/bby073 (2019).
Jiang, Z. et al. Quantitative proteomic analysis reveals that proteins required for fatty acid metabolism may serve as diagnostic markers for gastric cancer. Clin. Chim. Acta 464, 148–154. https://doi.org/10.1016/j.cca.2016.11.032 (2017).
Lucas, K., Gaines, L., Paris-Davila, T. & Nylander-French, L. A. Occupational exposure and serum levels of per- and polyfluoroalkyl substances (PFAS): a review. Am. J. Ind. Med. 66(5), 379–392. https://doi.org/10.1002/ajim.23454 (2023).
Panieri, E., Baralic, K., Djukic-Cosic, D., Buha, D. A. & Saso, L. PFAS molecules: a major concern for the human health and the environment. Toxics https://doi.org/10.3390/toxics10020044 (2022).
Cao, Y. & Ng, C. Absorption, distribution, and toxicity of per- and polyfluoroalkyl substances (PFAS) in the brain: a review. Environ. Sci. Process Impacts 23(11), 1623–1640. https://doi.org/10.1016/j.envres.2020.110690 (2021).
Steenland, K. & Winquist, A. PFAS and cancer, a sco** review of the epidemiologic evidence. Environ Res. 194, 110690. https://doi.org/10.1016/j.envres.2020.110690 (2021).
Gagliano, E., Sgroi, M., Falciglia, P. P., Vagliasindi, F. & Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 171, 115381. https://doi.org/10.1016/j.watres.2019.115381 (2020).
Antonacopoulou, A. G. et al. POLR2F, ATP6V0A1 and PRNP expression in colorectal cancer: new molecules with prognostic significance?. Anticancer Res. 28(2B), 1221–1227 (2008).
Lin, W. et al. Identification of a 6-RBP gene signature for a comprehensive analysis of glioma and ischemic stroke: cognitive impairment and aging-related hypoxic stress. Front Aging Neurosci. 14, 951197. https://doi.org/10.3389/fnagi.2022.951197 (2022).
Rosenbaum, J. T. et al. The role of the immune response in the pathogenesis of thyroid eye disease: a reassessment. PLOS ONE 10(9), e137654. https://doi.org/10.1371/journal.pone.0137654 (2015).
Nallu, R., Madhavan, P., Chirch, L. & Luthra, P. Thyroid eye disease due to immune reconstitution inflammatory syndrome as a consequence of antiretroviral therapy in the setting of AIDS. Case Rep. Endocrinol. 2020, 1728423. https://doi.org/10.1155/2020/1728423 (2020).
Lehmann, G. M., Feldon, S. E., Smith, T. J. & Phipps, R. P. Immune mechanisms in thyroid eye disease. Thyroid 18(9), 959–965. https://doi.org/10.1089/thy.2007.0407 (2008).
Sagiv, O. et al. Extraocular muscle enlargement and thyroid eye disease-like orbital inflammation associated with immune checkpoint inhibitor therapy in cancer patients. Ophthalmic Plast Reconstr. Surg. 35(1), 50–52. https://doi.org/10.1097/IOP.0000000000001161 (2019).
Chau, C., Shih, K. C., Chow, L. & Lee, V. Considerations for use of immune checkpoint inhibitors in cancer therapy for patients with co-existing thyroid eye disease. Ophthalmol Ther. 10(1), 5–12. https://doi.org/10.1007/s40123-020-00317-y (2021).
Yoon, J. S. & Kikkawa, D. O. Thyroid eye disease: from pathogenesis to targeted therapies. Taiwan J. Ophthalmol. 12(1), 3–11. https://doi.org/10.4103/tjo.tjo_51_21 (2022).
Sears, C. M. et al. Hearing dysfunction after treatment with teprotumumab for thyroid eye disease. Am. J. Ophthalmol. 240, 1–13. https://doi.org/10.1016/j.ajo.2022.02.015 (2022).
Liu, H. et al. Identification of hub genes associated with nonspecific orbital inflammation by weighted gene coexpression network analysis. Dis. Markers 2022, 7588084. https://doi.org/10.1155/2022/7588084 (2022).
Hu, J., Zhou, S. & Guo, W. Construction of the coexpression network involved in the pathogenesis of thyroid eye disease via bioinformatics analysis. Hum Genom. 16(1), 38. https://doi.org/10.1186/s40246-022-00412-0 (2022).
Huang, J. & Zhou, Q. Gene biomarkers related to Th17 cells in macular edema of diabetic retinopathy: cutting-edge comprehensive bioinformatics analysis and in vivo validation. Front. Immunol. 13, 858972. https://doi.org/10.3389/fimmu.2022.858972 (2022).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1), 27–30 (2000).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28(11), 1947–1951 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51(D1), D587–D592 (2023).
Funding
Financial support was provided by the National Natural Science Foundation of China (30772824,81574031); Key Laboratory of TCM Prevention and Treatment of Ent Diseases of Hunan Province (2017TP1018); Changsha Science and Technology Plan Project (K1501014-31, KC1704005); Central government financial support for the construction of local universities (2018–2019); State Administration of Traditional Chinese Medicine Key Discipline of Ophthalmology construction project; Key discipline construction project of TCM Five Senses Science in Hunan Province; Hunan Graduate Research Innovation Project (CX20220780); "Yifang" Graduate Innovation Project, Hunan University of Chinese Medicine (2022YF01).
Author information
Authors and Affiliations
Contributions
Z.W. drafted and revised the manuscript. Y.G. and L.C. were in charge of data collection. Q.P. and X.Y. conceived and designed this article, in charge of syntax modification and revised of the manuscript. All the authors have read and agreed to the final version manuscript. All authors have read and approved this manuscript to be considered for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Z., Gao, Y., Cao, L. et al. Purine metabolism-related genes and immunization in thyroid eye disease were validated using bioinformatics and machine learning. Sci Rep 13, 18391 (2023). https://doi.org/10.1038/s41598-023-45048-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-45048-9
- Springer Nature Limited
This article is cited by
-
Integration of bioinformatics and machine learning approaches for the validation of pyrimidine metabolism-related genes and their implications in immunotherapy for osteoporosis
BMC Musculoskeletal Disorders (2024)
-
A novel diabetic foot ulcer diagnostic model: identification and analysis of genes related to glutamine metabolism and immune infiltration
BMC Genomics (2024)
-
Causality of blood metabolites and metabolic pathways on Graves’ disease and Graves’ ophthalmopathy: a two-sample Mendelian randomization study
Endocrine (2024)