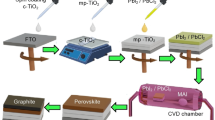
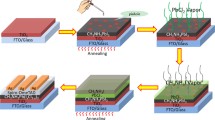
Abstract
Perovskite materials are fascinating candidates for the next-generation solar devices. With long charge carrier lifetime, metal-halide perovskites are known to be good candidates for low-light harvesting. To match the irradiance spectra of indoor light, we configured a triple-cation perovskite material with appropriate content of bromide and chloride (FA0.45MA0.49Cs0.06Pb(I0.62Br0.32Cl0.06)3) to achieve an optimum band gap (Eg) of \(\sim\)1.80 eV. With low photon flux at indoor condition, minimal recombination is highly desirable. To achieve such goal, we, for the first time, combined dual usage of antisolvent deposition and vacuum thermal annealing, namely VTA, to fabricate a high-quality perovskite film. VTA leads to compact, dense, and hard morphology while suppressing trap states at surfaces and grain boundaries, which are key culprits for exciton losses. With low-cost carbon electrode architecture, VTA devices exhibited average power conversion efficiency (PCE) of 27.7 ± 2.7% with peak PCE of 32.0% (Shockley–Queisser limit of 50–60%) and average open-circuit voltage (Voc) of 0.93 ± 0.02 V with peak Voc of 0.96 V, significantly more than those of control and the vacuum treatment prior to heat.
Similar content being viewed by others
Introduction
Energy demand has been dramatically increasing owing to population explosion and industrial expansion. Metal-halide perovskite-based materials are fascinating candidates for the next-generation solar devices. Because of exceptional light to electricity conversion characteristic and rapid increase of PCE over time to more than 25%, they are extremely promising for future commercial applications with PCE rivaling those of silicon solar cells1,2. Furthermore, perovskite solar cells (PSCs) have good optoelectronic properties such as high absorption over the visible spectrum3, low exciton binding energies4, low non-radiative recombination rates5, long charge carrier diffusion lengths in the \(\mu\)m range, and tunable energy band gaps (Eg) from 1.47 to more than 3.06 eV6,7. Interestingly, fabrication processes of PSCs are facile and economical with low consumption of energy due to high solubility at temperatures less than 100 °C8,9. Perovskite materials can be deposited by using several approaches, such as spin coating, vapor assisted solution deposition, thermal vapor deposition, inkjet printing, slot-die coating, and spray coating10,11,12,13. However, spin-coating deposition is a simple method for laboratory scale perovskite fabrication. One-step spin coating is a technique where precursor solution is directly spin–coated onto substrates14,15. Using antisolvent in one–step deposition, repeated cation do**, or adding some additives could be used to obtain large grain size, along with uniform and dense perovskite films10,16. Interestingly, Eg of perovskite-based materials with ABX3 structure can be tuned by substitution engineering at any site (A, B, and/or X), which is an advantage of perovskite technology, enabling several applications such as light emitting diodes (LEDs), photodetectors (PDs), and solar cells for both outdoor and indoor conditions. Up until now, researchers have paid a lot of attention to photovoltaics for low light that can be applied to portable electronics and wireless telecommunication technologies because of the rise of Internet of Things (IoTs)17,18,19. Perovskite–based materials are widely tunable in terms of energy band gaps, allowing a variety of Eg values each for an optimal performance under a specific light condition20,21. As indoor light sources provide different spectrum outputs compared to those of sunlight; according to computational calculation21,22, the well-fit energy band gap for indoor applications ranges from 1.8 to 1.9 eV, which can be achieved by partial substitution of A-site with cesium (Cs), methylammonium (MA), and/or formamidinium (FA) and/or X-site via iodine (I), bromine (Br), and/or chlorine (Cl)22,23,24,25,26. Cheng, R. et al. reported energy band gap tuning by the introduction of Br and Cl into pristine MAPbI3 perovskite. The Eg can be enlarged from 1.61 to 1.80 eV (MAPbI2−xBrClx). Moreover, the addition of chlorine reduces trap-state density, halide migration, and non-radiative recombination while improving crystallization, resulting in high open circuit voltage (Voc) of 1.028 V and PCE of 36.2% under 1000 lux fluorescent light21. Cheng, R. et al. demonstrated that perovskite solar cell performance was improved, particularly for indoor light applications by chlorine additive. The chlorine do** contributes to higher extraction capability at the perovskite/hole transport layer interface, which is attributed to lower defects19. With proper addition of Br, the grain sizes of perovskite were enlarged, suppressing non-radiative recombination. Moreover, the stability was improved by the formation of a pseudo-cubic phase and PCE of 34.5% was realized27. Apart from the perovskite absorber layer, electron transport layer (ETL) also plays important roles. Ann, M. H. et al. reported that compact TiO2 (c-TiO2) layer was more efficient for indoor light applications than mesoporous TiO2 (m-TiO2) layer owing to the high density of interfacial traps from using m-TiO2, although m-TiO2 was better for one sun condition28. Dagar, J. et al. also reported tin oxide (SnO2) as the electron transport layer for perovskite solar cell tested under indoor illumination, showing PCE of 21.3% at 400 lux29. From previous studies, the trap states at the interfaces and/or the grain boundaries are the main sources of non-radiative recombination22,23,28. Especially for low light applications, trap-state density is of great importance as there are less photo-generated charges under indoor light environment. To circumvent this problem under one sun, researchers have paid attention to the application of vacuum treatment to boost nucleation during crystallization and remove residual solvents without using antisolvent30,31,32. Li, X. et al. applied vacuum treatment to fabricate FA0.81MA0.15PbI2.51Br0.45 in DMSO-GBL solvent system and put the wet film under a vacuum environment for a few seconds to promote DMSO-PbI1.7Br0.3-(FAI)0.85(MABr)0.1 intermediate phase by eliminating solvent. The intermediate phase could delay crystal growth, leading to larger grain size and a high PCE of 20.5% under one sun30. In another study, low band gap FA0.8MA0.2Sn0.5Pb0.5I3 was deposited by applying vacuum-assisted growth control (VAGC) instead of antisolvent technique; the wet spin-coated films were placed under vacuum at 10 Pa for 10 s and further annealed under N2 atmosphere, resulting in smooth surfaces, no pin holes, large columnar grain orientations, fast transportation of charge carriers, and improved charge-carrier lifetime32. Zhang, J. et al. fabricated quasi-2D PEA2MAn-1PbnI3n+1 film by spin coating and vacuum treatment of the wet film to create uniform dispersion of different-n-value nanoplates to boost nucleations and limit the grain sizes by fast evaporation of residual solvents. The vacuum-treated films exhibited high fill factor (FF) of 82.4% and PCE of 18.04%31. Bi, D. et al. reported that perovskite solution (FA0.9Cs0.1PbI3) was added with molecular modulators (S, N, and SN) and fabricated by the one-step deposition method and then applied with a short vacuum treatment after spin coating to remove N, N-dimethylformamide (DMF) without using antisolvent to encourage fast crystallization of the intermediates. As a result, they achieved a PCE of over 20% with an active area of 1 cm233. Vacuum treatment was also applied to two-step deposition during the perovskite formation when MAI was dropped onto a PbI2 film, resulting in rapid solvent removal and a supersaturated state where a tremendous amount of nuclei are formed and simultaneously grown under the competitive pressure from neighbor nuclei; as a result, compact and smooth perovskite films with high hardness and thermal stability were produced34. Moreover, vacuum process can be applied during thermal annealing. **, and open-circuit voltage map** were performed by conductive atomic force microscope (C-AFM) via Park NX10 using a conductive probe (ANSCMPC, k = 0.036 N/m, and resonance frequency = 15 kHz). The measurement setup was done with a contact force setpoint of 1.8 nN and a scan speed of 2.5 μm/s. The sample biases are set to 0 V for the short circuit current map** and 0.6 V (forward bias) for the open circuit voltage map** while being excited with the microscope's white light with the power 0.2 mW/cm2 in ambient air (~70% RH) at room temperature (~25 °C).
One-sun illumination (100 mW/cm2) was provided by 7520-LED light source with LSS-7120 LED controller (VeraSol). 4 W LED 6500 K (Philipe, E27, cool daylight) was used as indoor light source. The light intensity was calibrated by Si diode (Hamamatsu S1133). The active area of each cell is 0.04 cm2. The photocurrent density–voltage (J-V) curves were measured from 1.00 V to −0.10 V under indoor light at 1000 lux (0.31 mW/cm2) and 1.10 V to −0.10 V under one sun with a scan step of 0.02 V and a delay time of 500 ms. The measurement was done under ambient air at room temperature without any encapsulation. The device stability was measured every two days for devices with encapsulation under ambient air at room temperture and under both 1000 lux and one sun; the devices were kept under 1000 lux for 8 h/day.
Results and discussion
The XRD patterns of perovskite films fabricated with and without VTA are illustrated in Fig. 2A. We found full transformation into perovskite thin films since PbI2 and hexagonal non-perovskite phase (δ-phase) peak could not be detected36,39. The XRD peak of our samples located at 14.5° corresponds to the crystallographic plane of (100), which is cubic phase40. The peak positions of perovskite are shifted to higher 2\(\uptheta\) degrees compared with our reference, which is around 14.0°40. According to Bragg’s law,
The increase in \(\uptheta\) indicates a decrease in d-spacing (d), accompanying partial substitution of larger I with smaller Br and/or Cl. Furthermore, according to the full width at half maximum (FWHM), the crystallite sizes were calculated by using the Scherrer equation at the (100) peaks. The average crystallite sizes are 48.1, 41.7, and 46.9 nm for control, VAC, and VTA, respectively. The crystallite sizes of all conditions are quite similar. However, the average crystallite size of control is the largest and that of VAC is the smallest. The average crystallite size of VTA is between those of control and VAC. The morphology of perovskite surfaces are examined by SEM (Fig. 2B–D) and atomic force microscopy (AFM) (Fig. 2F–H). Interestingly, we find that VTA perovskite formation is the most compact with the smallest roughness among the three with an average grain size of 265.3 ± 97.3 nm. While VAC results in the smallest average grain sizes of 218.5 ± 67.1 nm with many pinholes and high roughness. In contrast, the control condition has the biggest grain with an average grain size of 300.5 ± 121.7 nm along with medium roughness. VTA strikes the right balance, achieving good grain size and low roughness. SEM cross section images of all conditions are exhibited in Fig. S1. The control shows clear vertical grain boundaries, while VTA has the same trend with control. In contrast, the perovskite film processed with VAC shows randomly stacked small-size grains with pinholes. Vertical grains are beneficial, causing less recombination as the photogenerated carriers can pass through the active layer and reach the corresponding charge carrier extraction interfaces. VAC and VTA films are thinner when compared to the control film using the same concentration of perovskite solution, suggesting dense formation with vacuum. We hereby propose a mechanism based on a modified LaMer model16. In general, the crystal formation is divided into three phases. In phase I, the precursor concentration of the solution increases and reaches supersaturation (C*). In phase II, nuclei are formed when the precursor concentration exceeds C*. In phase III, nucleation stops and crystal growth pursues when concentration is between the saturation concentration (Cs) and C*. For the control sample, the triple-cation perovskite precursor solution is first spin-coated on top of ETL and antisolvent is dropped onto the rotating substrate to evaporate solvent, resulting in C* and nuclei generation. The concentration moderately reaches the C* region, resulting in a number of nuclei as primary nuclei. With thermal annealing after spin coating, the nucleation is stopped as the C* and Cs levels rise with temperature. While concentration is between C* and Cs (phase III), heat additionally helps remove DMSO from the intermediate phase30, resulting in crystal growth with large grain sizes as seen in many reports6,41,42,43,44. The film becomes shiny brown during the annealing process. For VTA sample, antisolvent is used to induce some amounts of nuclei called as primary nuclei similar to that of control. After antisolvent, the sample was thermally annealed in a vacuum chamber, which initiates secondary nuclei by fast evaporation at the surface30,31,32. In this step, we hypothesize that there are two concurring events; primary nuclei grow vertically and horizontally due to heat similar to that of control while C* at the surface creates secondary nuclei, which then grow due to heat to fill up the gaps between crystals of the primary nuclei. Smaller grains can be seen between larger grains as shown in Fig. 2D, resulting in smooth and compact morphology. The film becomes shiny brown in this vacuum annealing process. For antisolvent plus vacuum condition, antisolvent induces some amounts of nuclei as discussed previously. After antisolvent, the sample is moved to a vacuum chamber without heat. In this step, C* at the surface mostly creates secondary nuclei while reducing the amount of solvent without crystal growth as, without heat, concentration remains at C*. The film becomes hazy without brown color in this process. In the last step, heat is applied for 30 min; with removal of DMSO in the intermediate state, both primary and secondary nuclei grow at the same time with limited remaining solvent and therefore less degree of freedom for crystal formation. As a result, the VAC film shows much smaller grain without interconnection, as there is not enough activation energy to induce the desired transition process and facilitate compact grain growth44,45, leading to rough morphology as seen in Fig. 2C. The film becomes hazy with brown color in the annealing process. The average grain size of control, VAC, and VTA samples were shown in Fig. 2E. The graphically proposed mechanism was exhibited in Fig. 3 and the modified LaMer model of control, VAC, and VTA was shown in Fig. S2. With hardness measurement as shown in Table S1, the vacuum processes in both VTA and VAC result in mechanically harder films as compared to the control in agreement with previous publication34.
The absorption coefficients and the Tauc plots of perovskite films are shown in Fig. 4A and 4B. Because of different thicknesses among control, VAC, and VTA samples, the absorption coefficients46 were calculated for comparison. The VAC sample shows highest absorption coefficient as the film is rough and scatters more light47. Using the suitable amount of I, Br, and Cl, the absorption edges of all conditions are ~ 712 nm, exhibiting the band gap close to 1.80 eV, located at the lower range of optimal values for low light applications (1.80 − 1.90 eV). The similar values are expected since the physical changes due to vacuum should not affect chemical composition. These results are consistent with external quantum efficiency (EQE) as shown in Fig. 4C. The EQE measurement is used to measure the ratio of generated electrons to given photons at a specific wavelength of light excitation. The generated electrons only occur in the light absorption range as expected.
The steady-state photoluminescence (PL) spectra are shown in Fig. 4D to evaluate the charge transfer dynamics of perovskite films. The perovskite films were fabricated on FTO/SnO2 substrates. For VTA, the PL intensity is the strongest, indicating lower non-radiative recombinations36 because of the most compact film and less grain boundaries. In the PL spectra, VAC film shows the lowest PL intensity possibly due to high grain boundaries and pin holes, which contribute to non-radiative recombination. The PL peak of the VAC case shows sign of phase separation with Br-rich peak near 720 nm and I-rich peak near 770 nm. The VTA peak position is blue-shifted compared to that of control, signaling decreased spontaneous radiative recombination caused by trap states on the surface and/or grain boundariesFull size image
Furthermore, the short circuit current map (Isc map) was done to evaluate charge conductivity pixel by pixel at zero bias under 0.2 mW/cm2 illumination. Figure 7D–F show the Isc map on the surfaces. The VTA sample has the highest Iavg of 48.4 ± 37.1 pA due to robust formation as compared to 46.5 ± 42.4 pA and 46.0 ± 27.6 pA from VAC and control, respectively. Naturally, the trend of average currents from Isc maps is in line with Jsc results from Table 1. The Isc maps clearly illustrate high conductivity at perovskite cores for VTA sample, indicating superior charge transport52 not at the boundaries as observed in the Voc map. The poor Jsc results in VAC devices as compared to the relatively good Iavg from c-AFM could possibly be explained by large morphological distribution, as observed from rough SEM surface in Fig. 2C along with high roughness in Fig. 2G.