Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia. It is associated with a higher risk of important adverse health outcomes such as stroke and death. AF is linked to distinct electro-anatomic alterations. The main tool for AF diagnosis is the Electrocardiogram (ECG). However, an ECG recorded at a single time point may not detect individuals with paroxysmal AF. In this study, we developed machine learning models for discrimination of prevalent AF using a combination of image-derived radiomics phenotypes and ECG features. Thus, we characterize the phenotypes of prevalent AF in terms of ECG and imaging alterations. Moreover, we explore sex-differential remodelling by building sex-specific models. Our integrative model including radiomics and ECG together resulted in a better performance than ECG alone, particularly in women. ECG had a lower performance in women than men (AUC: 0.77 vs 0.88, p < 0.05) but adding radiomics features, the accuracy of the model was able to improve significantly. The sensitivity also increased considerably in women by adding the radiomics (0.68 vs 0.79, p < 0.05) having a higher detection of AF events. Our findings provide novel insights into AF-related electro-anatomic remodelling and its variations by sex. The integrative radiomics-ECG model also presents a potential novel approach for earlier detection of AF.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia. It is characterized by an irregular heart rhythm and often abnormally rapid heart rate. The most common complications of AF are increased risk of stroke, heart failure, and death1. These complications may be mitigated by early AF detection and initiation of appropriate treatments, such as anticoagulation and rate control therapies.
Cardiac structure and electrical activity are two important, inter-linked aspects of cardiac health and disease. The cardiac conduction system is complex and depends on the global and local structure of the cardiac chambers. The occurrence of AF is linked to distinct electro-anatomic cardiovascular remodeling2.
Electrical recordings of the heart such as the 12-lead electrocardiograms (ECG) provide indications of cardiovascular health. The ECG is a dynamic physiological signal that represents the electrical activity of the heart. It is widely used to identify patterns or abnormalities in cardiac rhythms and waveforms. ECG recordings are the main clinical tool for AF diagnosis3. The best indicators are the absence of the p-wave degenerating into small magnitude fibrillatory waves and the irregularity of R–R intervals indicating irregular conduction of atrial impulses through the atrioventricular (AV) node to the ventricles. The study of the QRS complex, a combination of the Q wave, R wave and S wave that represents ventricular depolarization, might also add some information by analyzing the height of the amplitude or the size of the interval. But the latter indicator might have normal values even when the AF is present4. Furthermore, AF frequently occurs intermittently with the characteristic AF-defining features only apparent when an individual experiences a paroxysm of AF. Whilst paroxysmal AF is more challenging to diagnose, it confers the same adverse risks as individuals continuously in an AF rhythm5.
Emerging deep learning approaches have shown promise in quantifying complex patterns in cardiac electrical activity6,7. However, there is room for improvement. For patients with undiagnosed AF, ischemic stroke may be the first clinical manifestation of the condition. AF is detected for first time in approximately one-fourth of patients presenting with ischemic stroke8,9. Early detection of AF may enable early intervention and prevention of ischemic stroke.
There are numerous conference challenges, particularly organized by Physionet, which aim to address early detection using machine learning techniques10. In spite of the successful results, the existing works in the literature do not stratify by sex. This is an important consideration given significant sex differential patterns in AF highlighted in clinical papers. The estimated prevalence of AF is lower in women, whilst this may reflect genuine lower burden of AF in women it may also indicate under-diagnosis in this population11. Indeed, women with AF experience higher mortality and ischemic stroke and are less often prescribed anticoagulation treatments12.
Cardiovascular Magnetic Resonance Imaging (CMR) plays an important role in the diagnosis of complex cardiac diseases. Recently, the concept of radiomics has attracted significant attention in the cardiac imaging community13 due to its ability to quantify and analyse large pools of advanced imaging phenotypes, which are descriptive of complex shape, size, intensity or textural patterns. Preliminary results have shown the promise of CMR radiomics for AF discrimination14.
CMR radiomics extracts a large number of quantitative features using data characterization algorithms. These techniques are very promising for deeper image phenoty** of cardiac structure and tissue15.
The combination of imaging phenotypes and ECG features for AF detection has not been explored in the existing literature. Yet, such an approach may enable integration of complementary signals and hence improve AF detection by considering both anatomical and electrical alterations.
In this work, we aim to evaluate the feasibility of combining cardiac imaging with ECG features for AF detection considering sex-differential patterns. Integrated risk prediction models were built combining CMR radiomics and ECG parameters, separately for men and women. Morphological, temporal and non-linear features were extracted from the ECG waveforms. The study was performed using the UK Biobank resource, a large-scale health database publicly available under request. To our knowledge, it is the first time, that the combination of ECG and imaging are explored. The inclusion of radiomics allows a more precise information of the AF event and quantifies the complexity of cardiac structure and remodeling providing a complementary information additionally to the ECG test.
Related work
The related works can be divided into three categories according to the computation of the features: ECG features extracted from the waveforms with machine learning techniques, ECG features extracted from deep learning methods and hybrid frameworks that combines traditional ECG features with the ones extracted using deep learning algorithms.
Classical approaches were mainly based on morphological features of the ECG signal in time domain such as heartbeat, analysis of intervals and amplitudes of QRS, QT, PR and R–R16,17. Those studies, with satisfactory results, may be sensitive to the ECG noise. To alleviate this issue, the morphological features were computed in other domains such as in the frequency or time–frequency domain. Some examples of these features are power spectral density of the R-R intervals and frequency bands (e.g., ultra low , very low and low). Non-linear features were also considered as the model of the heart cannot be reduced to a linear function as it also involves a nonlinear contribution18,19. Some works combining ECG features in different domains are the following: Yin et al. proposed a multi-domain ECG feature extraction method20. The multi-domain features were composed of nonlinear and frequency domain features, which were used as input features to train and test an SVM classifier model. Zabihi et al. also proposed a multi-domain ECG feature extraction which included time-domain features, time–frequency, phase-space based on non-linear features and meta-level information21. Random forest classifier was applied for feature selection as well as for classification.
As the technology evolves, more data can be processed, and deep learning techniques emerge. Many works have applied deep learning for feature extraction using convolutional neural networks (CNN)22,23,24, long and short memory networks (LSTM)25,26 as well as their variants8.
Discussion
In this study we demonstrate the feasibility and clinical utility of using an integrative electro-anatomic model for AF diagnosis. We demonstrate the usefulness of these models in understanding phenotypic alterations that occur in AF. Importantly, we identified different electro-anatomical remodeling patterns in male and female patients with AF. Our findings indicate the usefulness of a more integrative approach to disease in women, who may have more subtle phenotypic alterations than men, particularly in the early disease stages.
As ECG is the main clinical tool for AF diagnosis, we expected ECG to have better results than radiomics alone, as was shown in the results for the men and general models. However, we found lower performance of the ECG model for women than men in AF. This behavior is clearly seen when the models are split into female and male subjects. The combination of ECG with radiomics predictors was able to improve the model performance among female subjects. Radiomics showed less added value for men, however the most relevant features selected by the Chi-Squared test were radiomics-based features, particularly from the left atrial. Although, it did not improve the model’s overall accuracy, this finding suggests that radiomics features may precede ECG changes in both men and women.
The underlying mechanisms of the sex differences in AF are incompletely understood. The main driving factors reported in the literature are higher body mass index, larger atria and ventricle size among males36,37,38. Notably, atrial enlargement has been linked to higher risk of incident AF39 and AF recurrence40. Moreover a study by van de Vegte et al. demonstrated that genetically susceptibility to AF increases indexed left atrial volumes and decreases LA ejection fraction41. On the other hand, these factors might also impact the interpretation of the ECG signal.
Our results suggest that women with AF have less overt ECG changes than men. Indeed, women have a higher heart rate at rest due to hormone effects, autonomic nervous system influences, and intrinsic properties of the sinus node. The P-wave is significantly shorter as well as the PR interval and the QRS duration. QT has also a more prolonged corrected interval in women42. As an example, prolonged QT interval possibly cause lower sensitivity for ECG in women with leading to false positive cases. Moreover, shorter P-waves with lower amplitude might make ECG recordings susceptible to noise and motion artifacts43. This means that the subtler radiomics feature changes are important for improving AF detection in women.
Due to the more pronounced ECG changes among male participants our model can differentiate between cases and controls with high accuracy using these features alone. Notably, radiomics features appear dominant in the combined models even for men. This suggests that radiomics features are more sensitive at picking up AF-related alterations and these changes may complement the information derived from the ECG.
We also performed an extension including only the patients with the diagnosed AF who were in sinus rhythm at time of their ECG. As expected, ECG was not able to distinguish between healthy and unhealthy participants. However, the inclusion of radiomics substantially improved the model performance, particularly in women. Importantly, increased atrial volume44 and atrial fibrosis45 might serve as a substrate for AF, and these alterations can be picked up by radiomics features. Although further information is needed to better describe the link between atrial radiomics features and biological precursors of AF.
The utility of artificial intelligence-based methods has been already demonstrated in the detection of AF, importantly sex differences are rarely addressed in these studies. The Apple Heart Study assessed the ability of an irregular pulse notification algorithm to identify AF in 419,297 (42% female) individuals46. Overall, 2161 (21% female) participants received a notification and 34% of cases were clinically confirmed from the total number of users detected by the smartwatch. In the study positive predictive value of an irregular pulse notification was 0.84 (95% CI, 0.76–0.92), supporting the ability of the algorithm to correctly identify atrial fibrillation, mainly among white male subjects. Notably, the datasets collected among smart device users rarely permit the assessment of sex differences, as man are more likely to own these devices in the first place47. AI applications are also used in the monitoring48, risk stratification49 and management of AF patients.
As a future work, we will extend this work to other cohorts to generalize the models and validate them to external data. With the inclusion of more data, we will also explore deep learning techniques combining all leads with the features that we identified in this work to improve the model accuracy. Moreover, we will also differentiate between certain types of atrial fibrillation to find phenotypes in each category instead of atrial fibrillation patients in general. We will also test the utility of the present model to predict incident AF.
Limitations
Our ascertainment of AF status relied on clinical diagnoses. A limitation of this approach is that we would not capture as yet clinically unrecognized AF cases. As a result, some of the participants labelled as controls in our study may have low burden or paroxysmal AF that is not yet clinically identified. The impact of such misclassification would be attenuation rather than spurious high performance of our models. Additionally, the models were not validated externally limiting the generalizability of our results.
Conclusions
In this study of the UK Biobank participants we demonstrated that an ECG-based model had lower accuracy to detect AF in female subjects compared to males. The inclusion of CMR radiomics combined with ECG increased the model performance in women. Especially CMR derived radiomics shape features of the LA had robust role in the betterment of our models, suggesting the critical role of atrial remodeling in the disease mechanism of AF. The main universal implication is that a combined approach of ECG and atrial imaging might lead to better assessment of female participants suspected of AF.
As a further layer of our analysis we selected prevalent AF patients with normal ECG tests, here, we found that all models got benefit from adding radiomics. But again, the clearest case was for women with the inclusion of radiomics with ECG features.
Methods
Population and setting
UK Biobank is a large-scale health database containing over a half million of participants aged between 40 and 69 years old and recruited across UK between 2006 and 2010. It is a powerful research resource including biomarkers, medical records, risk factors, clinical tests and physical measurements to study the most common and life-threatening diseases. The database is regularly updated with additional data, making it a potential source for research purposes. AF was detected through the Hospital Episode Statistics (HES) system, a database containing clinical details of all the admissions of the NHS hospitals in England, to provide a continuous follow-up of the participants.
Study design and data
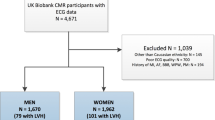
From the 495 prevalent AF cases in the UK Biobank cohort, we selected all the patients with AF who underwent both ECG and the CMR scan and the corresponding segmentation of the Left Ventricle (LV) and Right Ventricle (RV) cavities as well as the left and right atria were available (n = 383). To analyze the differences between sexes, we separated the data into female (n = 121) and male (n = 262) participants. Of these, 45 women and 49 men were in sinus rhythm at the time of their ECG recording. The healthy controls were defined as participants who were not diagnosed with AF and had a normal sinus rhythm on their ECG. For the healthy controls, we considered the first 2000 UK Biobank participants for computational purposes with ECG and CMR imaging. To avoid unbalanced models, the same number of healthy controls were randomly selected for each sex (Fig. 4).
Feature extraction
The features of ECG were extracted in temporal, morphological and non-linear domains. Radiomics features were computed from the LV and RV segmentations in end-systole (ES) and end-diastole (ED) phases from short- and long-axis cine CMR images. The radiomics of the atrias were computed from the long-axis images. In this section, we will explain in detail how the features were extracted.
Radiomics feature extraction
Radiomics features were extracted from the CMR images and the corresponding contours from three segmented ROIs: LV and RV cavities and LV myocardium in ED and ES in short axis. The segmentation of the ROIs was performed manually by expert cardiologists by defining the contours with points with a different label for each ROI using Cardiac MRI and CT Software (CVI42). The segmentations for each patient are exported in a single xml file containing the contour points for the RV, LV and MYO segmentations. In order to convert each contour into a binary mask, we developed an in-house software that transforms CVI42 contours into readable format contours50.
We also obtained the atrial segmentation using an automatic segmentation model based on a traditional U-Net architecture. First, a manual segmentation was performed by clinical experts in 764 datasets from Barts Heart Centre, UK51.Then, data augmentation techniques were used for generalizability of the model such as small rotations, random contrast adjustments and random intensity histogram shifting. The Adam optimizer was used with a learning rate of 0.0001 and 0.9 and 0.999 for first and second moments, respectively. The model was then trained with a batch size of 16 256 × 256 images with 100 epochs. The loss function used was cross entropy.
We computed the radiomics using the open-source python-based PyRadiomics library (version 2.2.0). To harmonize the images, the histogram matching technique was applied given a reference image. A binwidth of 25 was used to discretize the grey values of the image as it is the default parameter selected by pyradiomics. We extracted the relevant information present in the image by using three classes of features:
-
1.
First Order Features: are histogram-based features related to the distribution of the gray level values in the tissue, without focusing on their spatial relationships.
-
2.
Shape Features: describe geometrical properties of the organ, such as volume, diameter, minor/major axis and sphericity.
-
3.
Texture Features: are derived from images and allow quantification of spatial relationships among pixels.
The shape radiomics of all the ROIs both for the short axis and long axis were all considered. However, the first-order and textural features were only considered from the LV myocardium as the other ROIs included parts such as the papillary muscles that can alter the intensity signals within the ventricular and atrial cavities. Shape features derived from the LV myocardium, LV, RV and LA, RA were selected for the analysis, while first-order and textural features derived only from the LV myocardium were used. A total of 420 atrial radiomics features were computed in long axis where each ROI contained the same number of features of each type (ROI shape n = 24, ROI first-order n = 36, ROI texture n = 150). Additionally, 262 radiomics features both for short and for long axis were included from each CMR study (LV shape n = 26, RV shape n = 26, MYO shape n = 26, LV myocardium first-order n = 36, LV myocardium texture n = 148).
ECG feature extraction
We extracted the features of the ECG signals that are related according to literature with AF. We do not use the whole ECG signal as an input of the classification method to avoid overfitting. The ECG features for morphological, classical and non-linear features were computed using the open source code for ECG feature extraction in AF implemented in Matlab and mainly based on Physionet library52.
Firstly, classical ECG features were extracted based on morphological features in time domain including heartbeat intervals, analysis of QRS, QT, PR, R-R intervals and amplitude.
For robustness, morphological features in frequency domain were also extracted including power spectral density of the R-R intervals and frequency bands (ultra-low, very low, low and high frequency and Ratio of low- to high-frequency power).
Finally, non-linear features were also considered as the model of the heart is not only linear but also involves a nonlinear contribution. In this work, Poincaré Plot was used to extract non-linear features in ECG. Poincaré Plot is a 2D dimensional scatter plot where each point represents the RR interval as a function of the previous RR interval. The Poincaré analyzes quantitatively the shape of the plot which provides rich information of the behavior of the heart. For example, the plot for a patient with AF has a more circular shape than a healthy subject that is similar to a comet along the line of identity53.In order to determine the geometric appearance of the plot quantitively, some techniques such as ellipse fitting, correlation coefficient and histogram-based methods were implemented. Additionally, the Sample entropy was computed to measure the complexity of the time series54.
We proposed a multi-domain ECG feature extraction method including classical, non-linear and frequency domain features with a total number of 116 features. The second lead was used to extract the features as both old devices and the wearable devices are using a single lead. According to literature, the second lead provides the most valuable information55,56 including P, QRS and T waves. For that reason, it is the most used within the single-lead ECG works and the one with better results from 12-lead ECG recordings57.
Feature selection
Chi-squared test is applied to the features, and selects metrics statistically significantly linked to the outcome.
The Chi-squared test can be defined as given the data of two variables, we can get observed count O and expected count E. Chi-Square measures how expected count E and observed count O deviate from each other. The formulation is as follows:
A small p-value of the test statistic indicates that the corresponding features is dependent on the outcome, and it is an important feature. The statistical test returns each feature's importance score using the -log of the p-value. A large score value indicates that the corresponding feature is important. In our approach, the number of features selected was 30 as the model stabilizes after 30 features.
Statistical analysis
The experiments were conducted using the Matlab 2021b software. The correlation between ECG and radiomics was performed using Pearson’s correlation.
We used the fscchi2 function to apply the Chi-Squared test to select the most relevant features. A hierarchical model was built by combining radiomics with ECG to show the added value of incorporating radiomics features into the model for women, men and for both sexes. For comparison, we built the ECG and Radiomics-based models alone.
The models were trained with a Support Vector Machine (SVM) technique which has been widely used in cardiovascular risk predictions58,59 due to its numerous advantages such as computationally efficient and robustness for real-world applications as well as the ability to find non-linear relationships through the kernel trick.
The models were tested following a nested cross validation also known as double cross-validation, in order to minimize a biased evaluation of the accuracy of the model. Nested cross validation is widely employed in the machine learning field and was mainly developed to work with small datasets. Compared to standard cross validation techniques, nested cross validation can help in the reduction of overfitting and alleviate the limitation of optimistic biases, especially in relatively small samples. Varma and Simon et al., showed that nested cross validation methods provide an almost unbiased estimate of the true error compared to standard k-fold cross-validation particularly when used for both hyperparameter tuning and evaluation60,61,62. The method is divided into two loops: the inner loop is responsible for the selection of the best parameters, and the outer loop estimates the generalization accuracy62. This procedure splits the data into training and test folds k times in an outer loop. For each training fold, the hyperparameter optimization process is performed in an inner loop and returns the best parameters that minimize the error following the same procedure of partitioning and rotating the training fold into training and validation sets. Using this scheme, the test folds are never used to build the model, decreasing the possibility of overfitting. Notice that we have ten models trained with different partitions of the data not a single partition, making this procedure robust and reliable. Additionally, all the data has been used for testing making the performance measurements more reliable.
The hyperparameter optimization procedure was performed using greedy optimization which apply a brute force exhaustive search by trying each combination of each parameter. Five partitions are used for tuning the parameters of the SVM for each training fold in the inner loop (5-cross validation) and 10 cross-validation for the outer loop with partitions of 90% for training and 10% of testing in each outer fold. The summary of this procedure is shown in Fig. 5.
We computed to assess the performance of the models, the receiver operating characteristics (ROC) curve and area under the curve (AUC), as well as F1-score, accuracy, sensitivity and specificity over the test set. Additionally, Welch’s t-test was computed for group-wise comparisons. Several healthy partitions are randomly selected to show that the model does not depend on the selected data using different random seeds and we computed the ROC curve for each different partition of the healthy cohort.
To compare the models, a paired t-test on the distributions of AUC performances was performed to analyze the statistical significance in a nested cross validation framework63.
Data availability
The datasets generated and/or analysed during the current study are available online from the UK Biobank database, http://www.ukbiobank.ac.uk.
References
Chugh, S. S. et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation https://doi.org/10.1161/CIRCULATIONAHA.113.005119 (2014).
Pellman, J. & Sheikh, F. Atrial fibrillation: Mechanisms, therapeutics, and future directions. Compr. Physiol. https://doi.org/10.1002/cphy.c140047 (2015).
Hagiwara, Y. et al. Computer-aided diagnosis of atrial fibrillation based on ECG Signals: A review. Inf. Sci. https://doi.org/10.1016/j.ins.2018.07.063 (2018).
Fuster, V. et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. J. Am. Coll. Cardiol. https://doi.org/10.1016/j.jacc.2006.07.018 (2006).
Lip, G.Y.H., Li, F.L. & Hee, S. QJM paroxysmal atrial fibrillation.
Somani, S. et al. Deep learning and the electrocardiogram: Review of the current state-of-the-art. Europace 23, 1179–1191. https://doi.org/10.1093/europace/euaa377 (2021).
Li, Q., Liu, C., Oster, J. & Clifford, G. D. Signal processing and feature selection preprocessing for classification in noisy healthcare data. Mach. Learn. Healthc. Technol. https://doi.org/10.1049/PBHE002E_ch3 (2016).
Sposato, L. A., Chaturvedi, S., Hsieh, C. Y., Morillo, C. A. & Kamel, H. Atrial fibrillation detected after stroke and transient ischemic attack: A novel clinical concept challenging current views. Stroke https://doi.org/10.1161/STROKEAHA.121.034777 (2022).
Howlett, P. J. et al. Diagnosing paroxysmal atrial fibrillation: Are biomarkers the solution to this elusive arrhythmia?. BioMed Res. Int. https://doi.org/10.1155/2015/910267 (2015).
Lim, H. W., Hau, Y. W., Lim, C. W. & Othman, M. A. Artificial intelligence classification methods of atrial fibrillation with implementation technology. Comput. Assist. Surg. 21, 155–162. https://doi.org/10.1080/24699322.2016.1240303 (2016).
Ko, D. et al. Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nat. Rev. Cardiol. https://doi.org/10.1038/nrcardio.2016.45 (2016).
Kassim, N. A., Althouse, A. D., Qin, D., Leef, G. & Saba, S. Gender differences in management and clinical outcomes of atrial fibrillation patients. J. Cardiol. https://doi.org/10.1016/j.jjcc.2016.02.022 (2017).
Raisi-Estabragh, Z. et al. Cardiac magnetic resonance radiomics: Basic principles and clinical perspectives. Eur. Heart J.-Cardiovasc. Imaging. 21, 349–356 (2020).
Cetin, I., Petersen, S. E., Camara, O., González Ballester, M. Á. & Lekadir, K. Identifying alterations in the cardiac ventricles in atrial fibrillation: A radiomics approach. Int. J. Comput. Assist. Radiol. Surg. 14, 18 (2019).
Raisi-Estabragh, Z. et al. Associations of cognitive performance with cardiovascular magnetic resonance phenotypes in the UK Biobank. Eur. Heart J. Cardiovasc. Imaging. https://doi.org/10.1093/ehjci/jeab075 (2021).
Athif, M., Yasawardene, P. C. & Daluwatte, C. Detecting atrial fibrillation from short single lead ECGs using statistical and morphological features. Physiol. Meas. https://doi.org/10.1088/1361-6579/aac552 (2018).
Zong, W., Mukkamala, R. & Mark, R. G. A methodology for predicting paroxysmal atrial fibrillation based on ECG arrhythmia feature analysis. Comput. Cardiol. https://doi.org/10.1109/cic.2001.977607 (2001).
Acharya, U. R., Joseph, K. P., Kannathal, N., Lim, C. M. & Suri, J. S. Heart rate variability: A review. Med. Biol. Eng. Comput. https://doi.org/10.1007/s11517-006-0119-0 (2006).
Rizwan, A. et al. A review on the state of the art in atrial fibrillation detection enabled by machine learning. IEEE Rev. Biomed. Eng. https://doi.org/10.1109/RBME.2020.2976507 (2021).
Yin, L., Chen, F., Zhang, Q. & Ma, X. Arrhythmia classification based on multi-domain feature extraction. J. Phys. Conf. Ser. https://doi.org/10.1088/1742-6596/1237/2/022062 (2019).
Zabihi, M. et al. Detection of atrial fibrillation in ECG hand-held devices using a random forest classifier. Comput. Cardiol. https://doi.org/10.22489/CinC.2017.069-336 (2017).
Zubair, M., Kim, J. & Yoon, C. An automated ECG beat classification system using convolutional neural networks. in 2016 6th International Conference on IT Convergence and Security, ICITCS 2016. https://doi.org/10.1109/ICITCS.2016.7740310 (2016).
Kiranyaz, S., Ince, T. & Gabbouj, M. Real-time patient-specific ECG classification by 1-D convolutional neural networks. IEEE Trans. Biomed. Eng. https://doi.org/10.1109/TBME.2015.2468589 (2016).
Hsieh, C. H., Li, Y. S., Hwang, B. J. & Hsiao, C. H. Detection of atrial fibrillation using 1D convolutional neural network. Sensors (Switzerland) https://doi.org/10.3390/s20072136 (2020).
Schwab, P., Scebba, G. C., Zhang, J., Delai, M. & Karlen, W. Beat by beat: Classifying cardiac arrhythmias with recurrent neural networks. Comput. Cardiol. https://doi.org/10.22489/CinC.2017.363-223 (2017).
Faust, O. et al. Automated detection of atrial fibrillation using long short-term memory network with RR interval signals. Comput. Biol. Med. https://doi.org/10.1016/j.compbiomed.2018.07.001 (2018).
**a, Y., Wulan, N., Wang, K. & Zhang, H. Detecting atrial fibrillation by deep convolutional neural networks. Comput. Biol. Med. https://doi.org/10.1016/j.compbiomed.2017.12.007 (2018).
Andersen, R. S., Peimankar, A. & Puthusserypady, S. A deep learning approach for real-time detection of atrial fibrillation. Expert Syst. Appl. https://doi.org/10.1016/j.eswa.2018.08.011 (2019).
Fan, X. et al. Multiscaled fusion of deep convolutional neural networks for screening atrial fibrillation from single lead short ECG recordings. IEEE J. Biomed. Health Inform. https://doi.org/10.1109/JBHI.2018.2858789 (2018).
Smoleń, D. Atrial fibrillation detection using boosting and stacking ensemble. In Computing in Cardiology vol. 44 (2017).
Geelhoed, B. et al. Assessment of causality of natriuretic peptides and atrial fibrillation and heart failure: A Mendelian randomization study in the FINRISK cohort. Europace https://doi.org/10.1093/europace/euaa158 (2020).
Chyou, J. Y., Hunter, T. D., Mollenkopf, S. A., Turakhia, M. P. & Reynolds, M. R. Individual and combined risk factors for incident atrial fibrillation and incident stroke: An analysis of 3 million at-risk US patients. J. Am. Heart. Assoc. https://doi.org/10.1161/JAHA.114.001723 (2015).
Wong, J. A., Conen, D., Healey, J. S. & Johnson, L. S. B. Modifiable risk factors predict incident atrial fibrillation and heart failure. Open Heart. https://doi.org/10.1136/openhrt-2019-001092 (2020).
Esmeralda, R. P. et al. Prediction of incident cardiovascular events using machine learning and CMR radiomics. Radiology (2022)
Aizawa, Y., Watanabe, H. & Okumura, K. Electrocardiogram (ECG) for the prediction of incident atrial fibrillation: An overview. J. Atrial Fibrill. https://doi.org/10.4022/jafib.1724 (2017).
Kishi, S. et al. Race-ethnic and sex differences in left ventricular structure and function: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.114.001264 (2015).
McManus, D. D. et al. Longitudinal tracking of left atrial diameter over the adult life course: Clinical correlates in the community. Circulation https://doi.org/10.1161/CIRCULATIONAHA.109.885806 (2010).
Magnussen, C. et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts. Circulation https://doi.org/10.1161/circulationaha.117.028981 (2017).
Raisi-Estabragh, Z. et al. Left atrial structure and function are associated with cardiovascular outcomes independent of left ventricular measures: A UK Biobank CMR study. Eur. Heart J. Cardiovasc. Imaging. https://doi.org/10.1093/ehjci/jeab266 (2021).
Zacà, V. et al. Left atrial enlargement as a predictor of recurrences in lone paroxysmal atrial fibrillation. Can. J. Cardiol. https://doi.org/10.1016/S0828-282X(07)70841-3 (2007).
van de Vegte, Y. J., Siland, J. E., Rienstra, M. & van der Harst, P. Atrial fibrillation and left atrial size and function: A Mendelian randomization study. Sci. Rep. https://doi.org/10.1038/s41598-021-87859-8 (2021).
Boriani, G. et al. The effects of gender on electrical therapies for the heart: Physiology, epidemiology, and access to therapies. Europace https://doi.org/10.1093/europace/eux068 (2017).
Hossain, M. B., Bashar, S. K., Walkey, A. J., McManus, D. D. & Chon, K. H. An accurate QRS complex and P wave detection in ECG signals using complete ensemble empirical mode decomposition with adaptive noise approach. IEEE Access. https://doi.org/10.1109/ACCESS.2019.2939943 (2019).
Bertelsen, L. et al. Left atrial volume and function assessed by cardiac magnetic resonance imaging are markers of subclinical atrial fibrillation as detected by continuous monitoring. Europace https://doi.org/10.1093/europace/euaa035 (2020).
Sohns, C. & Marrouche, N. F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehz786 (2020).
Perez, M. V. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. https://doi.org/10.1056/nejmoa1901183 (2019).
Guo, Y. et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J. Am. Coll. Cardiol. https://doi.org/10.1016/j.jacc.2019.08.019 (2019).
Gopinathannair, R. et al. COVID-19 and cardiac arrhythmias: A global perspective on arrhythmia characteristics and management strategies. J. Intervent. Cardiac Electrophysiol. https://doi.org/10.1007/s10840-020-00789-9 (2020).
Inohara, T. et al. Association of of atrial fibrillation clinical phenotypes with treatment patterns and outcomes a multicenter registry study. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2017.4665 (2018).
euCanSHare. https://github.com/euCanSHare/dicom2nitfi.
Petersen, S. E. et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reson. 19, 1–19 (2017).
Andreotti, F., Carr, O., Pimentel, M. A. F., Mahdi, A. & de Vos, M. Comparing feature-based classifiers and convolutional neural networks to detect arrhythmia from short segments of ECG. Comput. Cardiol. (IEEE Computer Society) https://doi.org/10.22489/CinC.2017.360-239 (2017).
Henriques, T. et al. Nonlinear methods most applied to heart-rate time series: A review. Entropy https://doi.org/10.3390/e22030309 (2020).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public Health. https://doi.org/10.3389/fpubh.2017.00258 (2017).
da Luz, E. J. S., Schwartz, W. R., Cámara-Chávez, G. & Menotti, D. ECG-based heartbeat classification for arrhythmia detection: A survey. Comput. Methods Programs Biomed. https://doi.org/10.1016/j.cmpb.2015.12.008 (2016).
Murat, F. et al. Review of deep learning-based atrial fibrillation detection studies. Int. J. Environ. Res. Public Health. https://doi.org/10.3390/ijerph182111302 (2021).
Baalman, S. W. E. et al. A morphology based deep learning model for atrial fibrillation detection using single cycle electrocardiographic samples. Int. J. Cardiol. https://doi.org/10.1016/j.ijcard.2020.04.046 (2020).
Discrete cosine transform and support vector machines for classification cardiac atrial arrhythmia and cardiac normal. Int. J. Emerg. Trends Eng. Res. 8, 5400–5407. https://doi.org/10.30534/ijeter/2020/80892020 (2020).
Martinez-Alanis, M., Bojorges-Valdez, E., Wessel, N. & Lerma, C. Prediction of sudden cardiac death risk with a support vector machine based on heart rate variability and heartprint indices. Sensors (Switzerland) https://doi.org/10.3390/s20195483 (2020).
Varma, S. & Simon, R. Bias in error estimation when using cross-validation for model selection. BMC Bioinform. https://doi.org/10.1186/1471-2105-7-91 (2006).
Iizuka, N. et al. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet https://doi.org/10.1016/S0140-6736(03)12775-4 (2003).
Raschka, S. Model evaluation, model selection, and algorithm selection in machine learning performance estimation : Generalization performance vs . model selection. ar**v. (2018).
Izquierdo, C. et al. Radiomics-based classification of left ventricular non-compaction, hypertrophic cardiomyopathy, and dilated cardiomyopathy in cardiovascular magnetic resonance. Front. Cardiovasc. Med. https://doi.org/10.3389/fcvm.2021.764312 (2021).
Acknowledgements
This study was conducted using the UK Biobank resource under access application 2964. We would like to thank all the participants, staff involved with planning, collection and analysis, including core lab analysis of the CMR imaging data.
Funding
This study was supported by European Union's Horizon 2020 research and innovation programme (Grant No. 825903).This work was partly funded by the European Union's Horizon 2020 research and innovation programme under grant agreement no. 825903 (euCanSHare project) and grant agreement no. 965345 (HealthyCloud project). ZR-E recognises the National Institute for Health Research (NIHR) Integrated Academic Training programme which supports her Academic Clinical Lectureship post and was also supported by British Heart Foundation Clinical Research Training Fellowship No. FS/17/81/33318. L.S. received funding from the European Association of Cardiovascular Imaging (EACVI Research Grant App000076437). CM was supported by the Oxford NIHR Biomedical Research Centre. SEP acknowledges support from the 'SmartHeart' EPSRC programme grant (www. nihr.ac.uk; EP/P001009/1) and also from the CAP-AI programme, London's first AI enabling programme focused on stimulating growth in the capital's AI Sector. CAP-AI is led by Capital Enterprise in partnership with Barts Health NHS Trust and Digital Catapult and is funded by the European Regional Development Fund and Barts Charity. HV and BM received funding from the Ministry of Innovation and Technology NRDI Office within the framework of the Artificial Intelligence National Laboratory Program. SEP has also received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 825903 (euCanSHare project). SEP acknowledge the British Heart Foundation for funding the manual analysis to create a cardiovascular magnetic resonance imaging reference standard for the UK Biobank imaging-resource in 5000 CMR scans (www.bhf.org.uk; PG/14/89/31194). This project was enabled through access to the MRC eMedLab Medical Bioinformatics infrastructure, supported by the Medical Research Council (www.mrc.ac.uk; MR/L016311/1). The funders provided support in the form of salaries for authors as detailed above but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.Compliance with Ethical Standards.
Author information
Authors and Affiliations
Contributions
E.R. conducted the statistical analysis and Z.R., L.S., E.R. and K.L. analyzed the results. V.C. performed the atrial segmentations. E.R., L.S. and Z.R. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
SEP provides consultancy to and owns stock of Cardiovascular Imaging Inc, Calgary, Alberta, Canada.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pujadas, E.R., Raisi-Estabragh, Z., Szabo, L. et al. Atrial fibrillation prediction by combining ECG markers and CMR radiomics. Sci Rep 12, 18876 (2022). https://doi.org/10.1038/s41598-022-21663-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21663-w
- Springer Nature Limited
This article is cited by
-
Radiomics of pericardial fat: a new frontier in heart failure discrimination and prediction
European Radiology (2023)