Abstract
The role of miRNAs in cancer and their possible function as therapeutic agents are interesting and needed further investigation. The miR-26a-5p had been demonstrated as a tumor suppressor in various cancers. However, the importance of miR-26a-5p regulation in upper tract urothelial carcinoma (UTUC) remains unclear. Here, we aimed to explore the miR-26a-5p expression in UTUC tissues and to identify its regulatory targets and signal network involved in UTUC tumorigenesis. The miR-26a-5p expression was validated by quantitative real-time polymerase chain reaction (qPCR) using renal pelvis tissue samples from 22 patients who were diagnosed with UTUC and 64 cases of renal pelvis tissue microarray using in situ hybridization staining. BFTC-909 UTUC cells were used to examine the effects of miR-26a-5p genetic delivery on proliferation, migration and expression of epithelial-to-mesenchymal transition (EMT) markers. MiR-26a-5p was significantly down-regulated in UTUC tumors compared to adjacent normal tissue and was decreased with histological grades. Moreover, restoration of miR-26a-5p showed inhibition effects on proliferation and migration of BFTC-909 cells. In addition, miR-26a-5p delivery regulated the EMT marker expression and inhibited WNT5A/β-catenin signaling and expression of downstream molecules including NF-κB and MMP-9 in BFTC-909 cells. This study demonstrated that miR-26a-5p restoration may reverse EMT process and regulate WNT5A/β-catenin signaling in UTUC cells. Further studies warranted to explore the potential roles in biomarkers for diagnostics and prognosis, as well as novel therapeutics targets for UTUC treatment.
Similar content being viewed by others
Introduction
Urothelial carcinoma (UC) is a common cancer of the epithelium lining the urinary tract, among upper urinary tract cancers, including renal pelvis carcinoma and ureteral carcinoma as known as upper tract urothelial carcinoma (UTUC). Despite of a relatively uncommon malignancy in Western countries, UTUC represent more than 40% of UCs in Taiwan1. In addition, advanced UTUC is often associated with poor oncologic outcomes2. Therefore, UTUC is indeed a crucial health problem in Taiwan and develo** novel therapeutic and diagnostic strategies for the treatment is necessary.
MicroRNAs (miRNAs) are a class of small non-coding RNAs (19–22 nucleotides in length) that regulate gene expression at a post-transcriptional level via complementarily binding to their mRNA targets3. It is well known that miRNAs are involved in diverse biological processes, including cell differentiation, proliferation, and apoptosis3. Numerous miRNAs have been reported to display tumor suppressor activity in various cancers and may act as a potential target for treatment, including miR-26a4,5,6,7,8,9. There was a prostate cancer study showing that miR-26a was markedly reduced in cancer tissues and acted in concert to regulate genes that promote metastasis10. Moreover, studies had showed that miR-26a overexpression inhibits the tumor cell growth both in vitro and in vivo11. These findings support that miR-26a is a potential target of cancer therapy.
Epithelial-to-mesenchymal transition (EMT) is a process for the transformation of cancer cells from epithelial to mesenchymal types, which is clinically correlated with cancer metastasis and associated with recurrence and drug resistance in cancers3,12,13,14,15,16,17,18. Thus, suppressing EMT process could inhibit the migration and invasion during cancer progression. Additionally, there was study showing that reduced miR-26a expression is associated with lung metastasis and poor overall survival of osteosarcoma patients11.
To date, whether miR-26a (miR-26a-5p) regulates EMT process of UTUC cells and the molecular mechanisms involved remain unclear and need further investigation. The aims of this study were first to validate the miR-26a-5p expression using UTUC samples. Subsequently, we investigated the tumor suppressor function and mechanism using a renal pelvis transitional cell carcinoma cell line (BFTC-909 cells)3.
Results
miR-26a-5p expression was down-regulated in human UTUC tissues
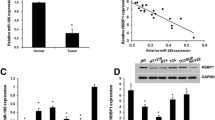
According to our previous study3, several microRNAs were down-regulated in UTUC tissues compared with adjusted normal controls, including miR-26a-5p. In this study, we first validated the miR-26a-5p expression in UTUC samples using qPCR assay and tissue microarray (TMA) using in situ hybridization staining (ISH). As shown in Fig. 1A, miR-26a-5p was significantly down-regulated in UTUC samples (n = 22:14). Thereafter, we next assessed miR-26a-5p expression in renal pelvis transitional cell carcinoma TMA which contained 64 tissue sections. As shown in Fig. 1B, miR-26a-5p was expressed around cytoplasmic area and the ISH immunosignals decreased progressively in advanced tumor grades. The intensity score was significantly lower in grade 3 tumors than grade 1 tumors (Fig. 1C). Taken together, these data imply the tumor suppressor role of miR-26a-5p in UTUC tumorigenesis.
Expression profiles of miR-26a-5p in human UTUC samples. (A) Down-regulation of miR-26a-5p in UTUC tumor tissues (n = 22) compared with adjacent normal control (n = 14) by RT-qPCR analysis. (B) In situ hybridization (ISH) analysis of miR-26a-5p expression in TMA of human renal pelvis carcinoma, including Grade 1 (G1; n = 23), Grade 2 (G2; n = 33) and Grade 3 (G3; n = 8) samples. (C) Quantification of miR-26a-5p expression levels from ISH staining results. Data represent the mean ± SEM. *P < 0.05 compared between the indicated groups.
Restoration of miR-26a-5p reduced BFTC-909 cells proliferation and migration
According to the ISH data, we further investigate the effects of miR-26a-5p restoration on UTUC cell proliferation and migration. Thus, we transfected miR-26a-5p mimics into BFTC-909 cells which is a well-known in vitro model of UTUC2,3. The proliferation was exam by Water-soluble tetrazolium (WST-1) assay and migration ability was exam by trans-well assay, respectively. As showed in Fig. 2, the miR-26a-5p restoration significantly reduced cell number (Fig. 2A) and migration ability (Fig. 2B,C). According to these results, we demonstrated that miR-26a-5p could serve as a tumor repressor in UTUC cell growth and migration.
Restoration of miR-26a-5p suppressed proliferation and migration of UTUC cells. BFTC-909 UTUC cells were transfected with miR-26a-5p mimics, followed by WST-1 and trans-well assays. NTC denotes non-transfection controls with scrambled mimetic treatment. (A) After mimetic transfection for 48 h, WST-1 assay was used to evaluate cell numbers. WST-1 value is directly proportional to cell number. (B) After mimetic transfection for 72 h, trans-well migration assay was performed to evaluate cell migration ability. (C) The crystal violet-stained cells are those penetrating the trans-well membrane. All data are shown as mean ± SEM from three independent experiments. *Indicates a P < 0.05 between the indicated groups.
Restoration of miR-26a-5p inhibited EMT processes
Epithelial mesenchymal transition processes has been implicated in carcinogenesis and confers metastatic properties12. Thus, we analyzed the effect of miR-26a-5p restoration in the EMT processes in UTUC while the E-cadherin, vimentin, α-SMA and fibronectin expression levels were assayed. As showed in Fig. 3, miR-26a-5p significantly restored cellular epithelial E-cadherin expression and inhibited α-SMA, fibronectin and vimentin expression. Moreover, the qPCR and western blot analyses similarly showed miR-26a-5p restoration significantly enhanced E-cadherin expression in mRNA and protein levels. Conversely, miR-26a-5p restoration reduced α-SMA, fibronectin and vimentin expression (Fig. 4). Together, these results indicated that miR-26a-5p might prohibit UTUC metastasis by regulating the EMT processes in UTUC cells.
miR-26a-5p overexpression inhibited expression of mesenchymal markers in UTUC cells. BFTC-909 UTUC cells were transfected with miR-26a-5p mimics for 48 h and subjected to immunofluorescent staining. (A) Representative images of cellular E-cadherin (green), vimentin (red), and nuclei (blue) were shown. (C) Alternatively, cellular fibronectin (green), α-SMA (red), and nuclei (blue) were immunofluorescently visualized. (B,D) The fluorescence intensities of epithelial marker E-cadherin as well as mesenchymal markers, vimentin, α-SMA and fibronectin, were quantified by counting 5–10 different fields per sample. Data are expressed as mean ± SEM (n = 3). *Indicates a P < 0.05 between the indicated groups. Original magnification: × 200.
miR-26a-5p overexpression inhibited expression of epithelial-to-mesenchymal transition markers in UTUC cells. BFTC-909 UTUC cells were transfected with miR-26a-5p mimics for 48 h and subjected to mRNA and protein measurements. Expression levels of epithelial marker E-cadherin and mesenchymal markers including vimentin, fibronectin, and α-SMA were assayed with qPCR (A) and western blot (B), respectively. GAPDH was used as loading internal control. (C) The blotting densitometry of protein levels in BFTC-909 cells. Data are expressed as mean ± SEM (n = 3). *Indicates a P < 0.05 between the indicated groups.
miR-26a-5p overexpression inactivation of WNT5A/β-catenin signaling in UTUC cells
The oncogenic mechanism underlying miR-26a-5p remains unclear. Because WNT5A/β-catenin signaling has been delineated to participate in the signal transduction of miR-26a-5p6,19,20, we compared the expression profiles of WNT5A/β-catenin signaling mediators between the UTUC cells with and without miR-26a-5p gene delivery. By using immunofluorescence staining, the data showed that miR-26a-5p restoration down-regulated the β-catenin and WNT5A expression (Fig. 5A,B) in UTUC cells. Furthermore, qPCR and immunoblot analyses confirmed that miR-26a-5p restoration reduced the mRNA and protein levels of β-catenin and WNT5A expression in UTUC cells (Fig. 6).
WNT5A/β-catenin signaling and downstream molecules were inhibited by the miR-26a-5p overexpression in UTUC cells. BFTC-909 cells were transfected with miR-26a-5p mimics for 48 h and subjected to immunofluorescent staining. Representative images for cellular distributions of (A) β-catenin (green), WNT5A (red), and nuclei (blue) were shown. (C) Alternatively, cellular distributions of MMP-9 (green), NF-κB p65 (red), and nuclei (blue) were immunofluorescently visualized. The fluorescence intensities of β-catenin and WNT5A (B), MMP-9 and NF-κB (D) were quantified by counting 5–10 different fields per sample. Data are expressed as mean ± SEM (n = 3). *Indicates a P < 0.05 between the indicated groups. Original magnification: × 200.
miR-26a-5p overexpression modulated expression of Wnt5A/β-catenin signaling mediators and downstream molecules in UTUC cells. BFTC-909 cells were transfected with miR-26a-5p mimics for 48 h and subjected to detection of mRNA and protein expression by using qPCR (A) and western blot (B), respectively. GAPDH was used as loading internal control. (C) The blotting densitometry of protein levels in BFTC-909 cells. Data are expressed as mean ± SEM (n = 3). *Indicates a P < 0.05 between the indicated groups.
miR-26a-5p overexpression inhibited NF-κB and MMP-9 expression in BFTC-909 cells
Previous studies have showed that WNT/β-catenin pathway inhibition has the potential to reduce cancer invasion and metastasis via regulating involves NF-κB/MMP-9 mediated EMT process15,21. Thus, we further investigated the regulatory role of miR-26a-5p in NF-κB (p65) and MMP-9 expression in UTUC cells. Immunofluorescence analysis again demonstrated that miR-26a-5p overexpression significantly inhibited the NF-κB and MMP-9 expression in BFTC-909 UTUC cells (Fig. 5C,D). These data were further confirmed by qPCR and western blot analyses (Fig. 6). These results demonstrated that miR-26a-5p delivery might block the EMT processes and prevent invasion and metastasis of UTUC cells. In summary, miR-26a-5p mechanistically inhibits the EMT process through suppression of WNT5A/β-catenin pathway during UTUC tumorigenesis. Thus, miR-26a-5p might be a good therapeutic target for UTUC treatment.
Discussion
Aberrant miRNA expression have been regarded a hallmark in all cancers, which disrupts the normal function of their targets and leads to the validation of tumor phenotypic transformation and metastasis as well as to drug resistance22. Thus, to fine out the gene regulatory effects of miRNAs may help exploit the potential treatment strategy in cancer diseases. There are studies suggesting that suppression of oncogenic miRNAs could become a reliable tool for improving the cancer therapy3,8,22.
miR-26a has been reported to act as tumor suppressor via targeting specific downstream genes in several human cancers, such as melanoma, thyroid, prostate cancer, laryngeal squamous cell and hepatocellular carcinoma6,23,24,25,26,27,28,29. However, the role of miR-26a-5p in UTUC tumorigenesis was largely unknown. In the present study, we report for the first time that miR-26a-5p expression was significantly down-regulated in human UTUC tissues compared with adjacent normal tissues. Moreover, the immunoexpression of miR-26a-5p decreased with higher T stage. The intensity score was lower in T3 tumors than T1 and T2 tumors (data not shown). In addition, the similar trends were found when compared to histological grade. This observation supports that high-grade tumor cells often constitutively express lower miR-26a-5p than low-grade tumor cells. Given that high histological grade is significantly related to aggressiveness and poor prognosis of tumor, our findings demonstrated that miR-26a-5p may play a tumor-suppressive role in the UTUC development. Consistent to our observation, the low miR-26a-5p expression has been earlier reported and identified as a poor prognostic marker in colorectal cancer due to poorer overall survival30. Therefore, it indicated a potential prognostic and therapeutic value of miR-26a-5p in UTUC. This is the first study to assay the miR-26a-5p expression in UTUC tissue samples. Moreover, our in vitro study showed that miR-26a-5p overexpression significantly suppressed the proliferation, migration and invasion of BFTC-909 cells. Furthermore, miR-26a-5p overexpression showed the inhibitory effect on EMT process which is associated with metastasis activity in UTUC cells. These results confirmed the inhibitory effect of miR-26a-5p on growth and metastasis in UTUC.
Metastasis is one of the major causes of mortality in UC patients31. Therefore, the inhibition of metastasis is an important issue in UC research, including UTUC. There are studies showing that inactivation of NF-κB and the inhibition of the expression of MMP-9, ultimately suppressing invasion and metastasis31,32. In addition, it has been reported that high MMP-9 expression levels are associated with clinically aggressive tumors and worse prognosis33,34,35. In the current study, we had provided the evidence of miR-26a-5p inhibited the NF-κB and MMP-9 expression. Thus, miR-26a-5p might regulate UTUC via suppressing invasion and metastasis.
It is well known that a single miRNA can affect multiple targets via distinct mechanisms36. It had been reported the therapeutic usefulness of inhibition of Wnt/β-catenin signaling in cancers27,37,38. Moreover, studies had revealed that miR-26a inhibited cell proliferation, metastasis, EMT, β-catenin and enhanced apoptosis, E-cadherin via mediating WNT5A in cancers6,27,39. The current study demonstrated the function of miR-26a-5p in regulating UTUC might mediate through inhibition of WNT5A/β-catenin signaling. Recently, various miRNA replacement therapies are currently in clinical trial demonstrates the great potential of this approach to treat cancer40. Therefore, miR-26a-5p may be a novel therapeutic small molecule against UTUC.
In conclusion, the present study provides the evidence to support the anticancer properties of miR-26a-5p and act as tumor suppressors in UTUC cells. The major findings of the present study are summarized as a diagrammatic depiction (Fig. 7): miR-26a-5p down-regulation in UTUC, which may block WNT5A/β-catenin signaling and inhibit EMT process. Thus, replacement therapy with miR-26a-5p represents a promising novel therapeutic strategy against UTUC.
Proposed mechanism for the effects of miR-26a-5p on WNT5A/β-catenin signaling in tumorigenesis of upper tract urothelial carcinoma (UTUC). Our findings revealed that miR-26a-5p gene delivery may induce simultaneous suppression of WNT5A and β-catenin expression and their downstream NF-κB and MMP-9 proteins. The suppression of WNT5A/β-catenin signaling axis by miR-26a-5p restoration could reverse the processes of epithelial-to-mesenchymal transition (EMT) of UTUC cells, including E-cadherin disruption, thereby suppressing their metastasis activity.
Materials and methods
Clinical specimens
All human UTUC tumor tissues (n = 22) and adjacent normal tissues (n = 14) were obtained in an UTUC cohort study in Kaohsiung Chang Gung Memorial Hospital, which was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB no. 201801703B1 and 101-4722B) and complied with the Helsinki Declaration. The written informed consent was obtained from all subjects or if subjects are under 18, from a parent/ legal guardian.
Human UTUC tissue microarray (TMA)
The human renal pelvis transitional cell carcinoma TMA containing 64 cases was purchased from US Biomax, Inc. (Kit no.KD642, Rockville, MD). The TMA slide was subjected to in situ hybridization (ISH) staining of miR-26a-5p, and quantified by pathologist according to the following rules. The labeling intensity was given a score from 1 to 4, corresponding to yellow, yellow–brown, brown and dark staining, respectively. The proportion of tumor cells with detectable cytoplasmic immunoreactivity for miR-26a-5p were also recorded using a 4-tier score, for 1 corresponding to < 30% tumor cells with positive staining, 2 for 30–60%, 3 for 60–80%, and 4 for > 80%. An expression index was defined as the product of these two scores mentioned above. Obviously, the index could range from 1 to 16, with 16 corresponding to > 80% tumor cells displaying dark (4) staining.
miR-26a-5p in situ hybridization
TMA slide was examined using in situ hybridization (ISH) staining. Tissue sections were stained with digoxigenin-labeled probe (sequence 5ʹ-AGC CTA TCC TGG ATT ACT TGAA-3ʹ) synthesized by BioTnA and complimentary to miR-26a-5p The expression level was measured by using a Biospot ISH detection kit (TASH01D, BioTnA, Kaohsiung, Taiwan). The signals were developed by DAB chromogen and documented under microscope as described41.
RNA isolation, reverse transcription and quantitative PCR (qPCR)
Total RNA was extracted by using Trizol® Reagent (Invitrogen, USA) according to the instruction manual as previously described3. To prepare a cDNA pool from each RNA sample, total RNA (10 ng) was reverse-transcribed using TaqMan MicroRNA reverse transcription kit (ABI, Cat. 4366596) according to the manufacturer’s instructions as previously described3. The primers are listed on Supplementary Table 1.
Cell culture and miR-26a-5p treatment
A UTUC cell line, clone BFTC-909, was cultured as described previously3. To observe the biomodulatory effect of miR-26a-5p on UTUC cell behaviors, the cells were stably transfected with miR-26a-5p mimics following the manufacturer’s instructions and the Guidelines for miRNA mimic and miRNA inhibitor experiments (QIAGEN, Hilden, Germany).
Cell proliferation assay
Cell proliferation assay was performed by using WST-1 assay kit (TaKaRa Cat. # MK400). All procedures were followed as previously described3.
Trans-well cell migration assay
Cell migration assay was performed using Trans-well chambers (Millicell, PIEP12R48). All procedures were followed as previously described3.
Western blot analysis
Cellular protein lysates were prepared by homogenization with 1× RIPA lysis buffer (Cell Signaling Technology, Billerica, MA). Protein concentrations were measured using a protein assay dye (Bio-Rad Laboratories, Hercules, CA). SDS-PAGE and immunoblotting analysis were performed as described previously3. The detecting antibodies were raised against E-cadherin (ab76055, abcam), vimentin (2707-1 epitomics), α-SMA (ab5694, abcam), fibronectin (ab2413, abcam), WNT5A (MA5-15502, Invitrogen), β-catenin (13-8400, Invitrogen), NF-κB (ab32536, abcam), MMP-9 (13667, CST), and GAPDH (GTX627408, GeneTex).
Immunofluorescent staining
BFTC909 cells were cultured in six-well glass slide chambers for 24 h and further transfected with miR-26a-5p mimics for 48 h. The cells were then fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, and blocked with 3% BSA for 30 min at room temperature. The fixed cells were then incubated with the primary antibodies against E-cadherin, vimentin, fibronectin, α-SMA, WNT5A, β-catenin, NF-κB, and MMP-9 at 4 °C overnight followed by visualization with Alexa Fluor 488- (green) or Alexa Fluor 595 (red)-conjugated secondary antibodies at room temperature for 1 h. Nuclei were counterstained with DAPI. The stained cells were mounted with a fluorescent mounting medium (Dako Cytomation) and observed under fluorescence microscopy (Olympus). The exposure gains and rates were consistent between samples. Fluorescent intensities were quantified on independent color channels by using Image J software (NIH, USA)3.
Statistical analysis
All values in the figures were expressed as mean ± standard error of the mean and analyzed by unpaired t test. P value < 0.05 was considered to be statistically significant.
References
Wu, Y. T. et al. Gender effect on the oncologic outcomes of upper urinary tract urothelial carcinoma in Taiwan. Int. Urol. Nephrol. https://doi.org/10.1007/s11255-020-02396-z (2020).
Luo, H. L. et al. Methylation of SPARCL1 is associated with oncologic outcome of advanced upper urinary tract urothelial carcinoma. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20071653 (2019).
Chung, Y. H. et al. MiR-30a-5p inhibits epithelial-to-mesenchymal transition and upregulates expression of tight junction protein claudin-5 in human upper tract urothelial carcinoma cells. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18081826 (2017).
Ursu, S. et al. Novel tumor suppressor role of miRNA-876 in cholangiocarcinoma. Oncogenesis 8, 42. https://doi.org/10.1038/s41389-019-0153-z (2019).
Zhang, L., Liao, Y. & Tang, L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J. Exp. Clin. Cancer Res. 38, 53. https://doi.org/10.1186/s13046-019-1059-5 (2019).
Shi, D. et al. MicroRNA-26a-5p inhibits proliferation, invasion and metastasis by repressing the expression of Wnt5a in papillary thyroid carcinoma. Onco Targets Ther. 12, 6605–6616. https://doi.org/10.2147/OTT.S205994 (2019).
Coronel-Hernandez, J. et al. Cell migration and proliferation are regulated by miR-26a in colorectal cancer via the PTEN-AKT axis. Cancer Cell Int. 19, 80. https://doi.org/10.1186/s12935-019-0802-5 (2019).
Gambari, R., Brognara, E., Spandidos, D. A. & Fabbri, E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: New trends in the development of miRNA therapeutic strategies in oncology (review). Int. J. Oncol. 49, 5–32. https://doi.org/10.3892/ijo.2016.3503 (2016).
Ji, W., Sun, B. & Su, C. Targeting microRNAs in cancer gene therapy. Genes (Basel). https://doi.org/10.3390/genes8010021 (2017).
Kato, M. et al. Regulation of metastasis-promoting LOXL2 gene expression by antitumor microRNAs in prostate cancer. J. Hum. Genet. 62, 123–132. https://doi.org/10.1038/jhg.2016.68 (2017).
Lu, J. et al. MiR-26a inhibits stem cell-like phenotype and tumor growth of osteosarcoma by targeting Jagged1. Oncogene 36, 231–241. https://doi.org/10.1038/onc.2016.194 (2017).
Heerboth, S. et al. EMT and tumor metastasis. Clin. Transl. Med. 4, 6. https://doi.org/10.1186/s40169-015-0048-3 (2015).
Du, B. & Shim, J. S. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. https://doi.org/10.3390/molecules21070965 (2016).
Pastushenko, I. & Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 29, 212–226. https://doi.org/10.1016/j.tcb.2018.12.001 (2019).
Yang, H. L. et al. Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J. Exp. Clin. Cancer Res. 38, 186. https://doi.org/10.1186/s13046-019-1196-x (2019).
Kumar, A., Golani, A. & Kumar, L. D. EMT in breast cancer metastasis: An interplay of microRNAs, signaling pathways and circulating tumor cells. Front. Biosci. (Landmark Ed.) 25, 979–1010 (2020).
Hill, C. & Wang, Y. The importance of epithelial-mesenchymal transition and autophagy in cancer drug resistance. Cancer Drug Resist. 3, 38–47. https://doi.org/10.20517/cdr.2019.75 (2020).
Huang, C. C. et al. Delta-like 1 homologue promotes tumorigenesis and epithelial-mesenchymal transition of ovarian high-grade serous carcinoma through activation of Notch signaling. Oncogene 38, 3201–3215. https://doi.org/10.1038/s41388-018-0658-5 (2019).
Li, S. et al. Effect of miR-26a-5p on the Wnt/Ca(2+) pathway and osteogenic differentiation of mouse adipose-derived mesenchymal stem cells. Calcif. Tissue Int. 99, 174–186. https://doi.org/10.1007/s00223-016-0137-3 (2016).
Li, Y. et al. Long non-coding RNA SNHG5 promotes human hepatocellular carcinoma progression by regulating miR-26a-5p/GSK3beta signal pathway. Cell Death Dis. 9, 888. https://doi.org/10.1038/s41419-018-0882-5 (2018).
Wang, J. et al. Circadian protein BMAL1 promotes breast cancer cell invasion and metastasis by up-regulating matrix metalloproteinase9 expression. Cancer Cell Int. 19, 182. https://doi.org/10.1186/s12935-019-0902-2 (2019).
Cui, M. et al. Circulating microRNAs in cancer: Potential and challenge. Front. Genet. 10, 626. https://doi.org/10.3389/fgene.2019.00626 (2019).
Li, Y. et al. MicroRNA-26a inhibits proliferation and metastasis of human hepatocellular carcinoma by regulating DNMT3B-MEG3 axis. Oncol. Rep. 37, 3527–3535. https://doi.org/10.3892/or.2017.5579 (2017).
Rhee, J. W. et al. NF-kappaB-dependent regulation of matrix metalloproteinase-9 gene expression by lipopolysaccharide in a macrophage cell line RAW 264.7. J. Biochem. Mol. Biol. 40, 88–94. https://doi.org/10.5483/bmbrep.2007.40.1.088 (2007).
Chang, L., Li, K. & Guo, T. miR-26a-5p suppresses tumor metastasis by regulating EMT and is associated with prognosis in HCC. Clin. Transl. Oncol. 19, 695–703. https://doi.org/10.1007/s12094-016-1582-1 (2017).
Rizzo, M. et al. Discovering the miR-26a-5p targetome in prostate cancer cells. J. Cancer 8, 2729–2739. https://doi.org/10.7150/jca.18396 (2017).
Zhao, S. et al. MiR-26a inhibits prostate cancer progression by repression of Wnt5a. Tumour Biol. 35, 9725–9733. https://doi.org/10.1007/s13277-014-2206-4 (2014).
Wu, Z. et al. MicroRNA-26a inhibits proliferation and tumorigenesis via targeting CKS2 in laryngeal squamous cell carcinoma. Clin. Exp. Pharmacol. Physiol. 45, 444–451. https://doi.org/10.1111/1440-1681.12890 (2018).
Qian, H., Yang, C. & Yang, Y. MicroRNA-26a inhibits the growth and invasiveness of malignant melanoma and directly targets on MITF gene. Cell Death Discov. 3, 17028. https://doi.org/10.1038/cddiscovery.2017.28 (2017).
Li, Y. et al. Tumor-suppressive miR-26a and miR-26b inhibit cell aggressiveness by regulating FUT4 in colorectal cancer. Cell Death Dis. 8, e2892. https://doi.org/10.1038/cddis.2017.281 (2017).
Qin, J. et al. Epigallocatechin-3-gallate inhibits bladder cancer cell invasion via suppression of NF-kappaBmediated matrix metalloproteinase-9 expression. Mol. Med. Rep. 6, 1040–1044. https://doi.org/10.3892/mmr.2012.1054 (2012).
Guarneri, C. et al. NFkappaB inhibition is associated with OPN/MMP9 downregulation in cutaneous melanoma. Oncol. Rep. 37, 737–746. https://doi.org/10.3892/or.2017.5362 (2017).
Cioroianu, A. I. et al. Tumor microenvironment in diffuse large B-cell lymphoma: Role and prognosis. Anal. Cell Pathol. (Amst.) 2019, 8586354. https://doi.org/10.1155/2019/8586354 (2019).
Abdollahi, A., Nozarian, Z. & Nazar, E. Association between expression of tissue inhibitors of metalloproteinases-1, matrix metalloproteinase-2, and matrix metalloproteinase-9 genes and axillary lymph nodes metastasis in patients with breast cancer. Int. J. Prev. Med. 10, 127. https://doi.org/10.4103/ijpvm.IJPVM_355_16 (2019).
Shang, S. et al. Macrophage ABHD5 suppresses NFkappaB-dependent matrix metalloproteinase expression and cancer metastasis. Cancer Res. 79, 5513–5526. https://doi.org/10.1158/0008-5472.CAN-19-1059 (2019).
Si, W., Shen, J., Zheng, H. & Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 11, 25. https://doi.org/10.1186/s13148-018-0587-8 (2019).
Ma, B., Fey, M. & Hottiger, M. O. WNT/beta-catenin signaling inhibits CBP-mediated RelA acetylation and expression of proinflammatory NF-kappaB target genes. J. Cell Sci. 128, 2430–2436. https://doi.org/10.1242/jcs.168542 (2015).
Sha, Y. L., Liu, S., Yan, W. W. & Dong, B. Wnt/beta-catenin signaling as a useful therapeutic target in hepatoblastoma. Biosci. Rep. https://doi.org/10.1042/BSR20192466 (2019).
Li, Y. et al. miR-26a-5p inhibit gastric cancer cell proliferation and invasion through mediated Wnt5a. Onco Targets Ther. 13, 2537–2550. https://doi.org/10.2147/OTT.S241199 (2020).
Hosseinahli, N., Aghapour, M., Duijf, P. H. G. & Baradaran, B. Treating cancer with microRNA replacement therapy: A literature review. J. Cell Physiol. 233, 5574–5588. https://doi.org/10.1002/jcp.26514 (2018).
Chung, Y. H. et al. BMP-2 restoration aids in recovery from liver fibrosis by attenuating TGF-beta1 signaling. Lab. Investig. 98, 999–1013. https://doi.org/10.1038/s41374-018-0069-9 (2018).
Acknowledgements
This study was supported by Grant CMRPG8H0391 from Kaohsiung Chang Gung Memorial Hospital, Kaohsiung Taiwan. Special thanks to all our group members, especially, the Genomics & Proteomics Core Laboratory, Department of Medical Research, Kaohsiung Chang Gung Memorial Hospital for technical supports and laboratory facilities.
Author information
Authors and Affiliations
Contributions
Y.H.C. and Y.T.C. wrote the main manuscript text and completed this study. Y.H.K. and W.C.T. edited the main manuscript. Y.C.S., Y.T.C., P.H.C. and Y.T.C. were involved in study sample collection. G.K.H. provided pathological interpretation and analysis. M.H.T. and P.H.C. conceived the study and critically revised the final manuscript. M.H.T. and P.H.C. were study supervisors. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, YH., Cheng, YT., Kao, YH. et al. MiR-26a-5p as a useful therapeutic target for upper tract urothelial carcinoma by regulating WNT5A/β-catenin signaling. Sci Rep 12, 6955 (2022). https://doi.org/10.1038/s41598-022-08091-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08091-6
- Springer Nature Limited