Abstract
Retinoid X receptors are members of the nuclear receptor family that regulate gene expression in response to retinoic acid and related ligands. Group 1 metabotropic glutamate receptors are G-protein coupled transmembrane receptors that activate intracellular signaling cascades in response to the neurotransmitter, glutamate. These two classes of molecules have been studied independently and found to play important roles in regulating neuronal physiology with potential clinical implications for disorders such as depression, schizophrenia, Parkinson’s and Alzheimer’s disease. Here we show that mice lacking the retinoid X receptor subunit, RXRγ, exhibit impairments in group 1 mGluR-mediated electrophysiological responses at hippocampal Schaffer collateral-CA1 pyramidal cell synapses, including impaired group 1 mGluR-dependent long-term synaptic depression (LTD), reduced group 1 mGluR-induced calcium release, and loss of group 1 mGluR-activated voltage-sensitive currents. These animals also exhibit impairments in a subset of group 1 mGluR-dependent behaviors, including motor performance, spatial object recognition, and prepulse inhibition. Together, these observations demonstrate convergence between the RXRγ and group 1 mGluR signaling pathways that may function to coordinate their regulation of neuronal activity. They also identify RXRγ as a potential target for the treatment of disorders in which group 1 mGluR signaling has been implicated.
Similar content being viewed by others
Introduction
Proper control of neuronal activity and synaptic transmission is critical for normal nervous system function, and accordingly, multiple neurotransmitters, neuromodulators, and signaling pathways have been found to participate in their regulation. However, neurons do not respond to these factors in isolation. To produce appropriate responses, neurons need to integrate multiple inputs.
Here we identify an interaction between two pathways known to play important roles in regulating neuronal and synaptic activity: (1) group 1 metabotropic glutamate receptors (mGluRs) and (2) the nuclear hormone receptor family member, retinoid X receptor gamma (RXRγ). Specifically, we found that animals lacking RXRγ exhibited impairments in group 1 mGluR-mediated synaptic transmission and synaptic plasticity, together with impairments in group 1 mGluR-mediated behaviors. These data suggest an interaction between these pathways in normal learning and memory and in the control of disease-relevant behaviors including Parkinson’s disease-related motor impairments, and Schizophrenia-related executive function.
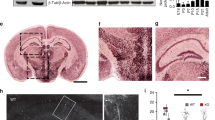
RXRγ is one of three retinoid X receptors (α, β, γ) found in mice and humans with partially overlap** expression patterns and functions. RXRs are members of the nuclear receptor superfamily and form heterodimers with other nuclear receptor family members that bind to discrete DNA sequences to regulate transcription in response to ligand binding (reviewed inCalcium imaging Calcium imaging was performed on proximal apical dendrites of CA1 pyramidal cells in acute hippocampal slice preparations as described previously96. A customized two-photon laser-scanning Olympus BX61WI microscope with a 60×/1.1 nA objective was used to detect Ca2+ signals. A Mai/Tai laser (Solid-State Laser, Mountain View, CA) tuned to 820 nm was used for excitation, and image acquisition and storage was controlled by Olympus Fluoview FV300 software (Olympus, Melville, NY). In the transfluorescence pathway, a 565 nm dichroic mirror was used to separate green and red fluorescence, passed through HQ525/50 and HQ605/50 emission filters, to eliminate transmitted or reflected excitation light (Chroma Technology, Rockingham, VT), and detected simultaneously by two photomultiplier tubes. Neurons were loaded with dyes through the patch pipette for 20 min before commencing image acquisition. Alexa Fluor-594 was used to outline neuronal dendritic structure, and Calcium Green-1 (100 µM in the patch pipette) to detect [Ca2+] changes. To measure [Ca2+] dynamics, fluorescence was collected by scanning at 4–5 Hz in a surface-scanning mode (XYT), or 1 kHz in XT mode, and averaged from specified structures to obtain F(t). Baseline fluorescence (F0) was calculated as the average of four images obtained immediately prior to bath application of 30 μM DHPG. Images of the same cells were obtained after 5, 10, 15 and 20 min of DHPG application. Normalized values for each cell at each time point were calculated by dividing the fluorescent signals at a given time point by the average fluorescent signal for that cell obtained prior to drug treatment (F0). No EGTA was added to the internal solution for [Ca2+] imaging. Hippocampi were homogenized by sonication in 50 mM Tris pH 7.4/2% SDS followed by incubation at 95 °C for 5 min. Extracts were run in duplicate and resolved by denaturing PAGE and blotted to nylon membrane (Immobilon-FL, Millipore). Blocking and antibody incubations were for 1 h at room temperature in Tris-buffered saline, 0.05% tween 20, 3% non-fat dry milk. Washes were carried out TBS-Tween without milk. Proteins were detected using commercially available antibodies against mGluR1 (Millipore AB1551), mGluR5 (Millipore AB5675), and α-tubulin (Sigma T9026) in conjunction with dye conjugated anti-mouse or rabbit secondary antibodies (Li-COR, Lincoln, NE). Blots were scanned and integrated band intensities determined using an Odyssey infrared imaging system. mGluR1 or mGluR5 band intensities were normalized to the band intensity for tubulin in that lane as a loading control and data were presented as percent of the average normalized control value. Total hippocampal RNA was prepared using Trizol Reagent and first strand cDNAs were prepared using the Superscript III first strand cDNA synthesis supermix for Q-PCR (Invitrogen) as per manufacturers instructions. Quantitect primer pairs were purchased from Qiagen. All primer pairs showed a linear response of Log(template dilution) vs. cycle threshold for template dilutions over 5 orders of magnitude. Optimal annealing temperatures, template dilutions and corresponding efficiencies were determined experimentally for each primer pair, and melting curves determined for each reaction showed a single peak under these conditions. Relative expression levels were calculated for each sample using the experimentally determined efficiency and the number of cycles to reach a florescence threshold set manually within the linear portion of a plot of the Log(florescence intensity) vs. cycle number. Values were normalized to those obtained using a primer pair specific for GAPDH run in parallel for each sample. Brains were fixed by transcardial perfusion in 4%PFA dissolved in 100 mM phosphate buffer pH 7.4. 30 μM sections were cut using a Vibratome slicer. Floating sections were permeablized for 30 min at 4 °C in Tris-buffered saline + 0.2% triton and blocked for 1 h at room temp in Tris-buffered saline + 10% fetal bovine serum. Primary antibody incubations were carried out overnight at 4 °C in blocking buffer, secondary antibody incubations were carried out for 1 h at room temperature also in blocking buffer, and washes were TBS + 0.2% triton. Primary antibodies were from Millipore as above, and an Alexa-568 anti-rabbit from Invitrogen was used as a secondary. Stained sections were mounted in Fluorsave aqueous mounting medium (Calbiochem) and images were obtained by confocal microscopy (Olympus Fluoview FV1000 and associated software). Behavior in a novel open field was assessed using Plexiglas activity chambers (model ENV- 520; Med Associates, St. Albans, Vermont) (43.2 cm long × 43.2 cm wide × 30.5 cm high). Mice were placed in the open field and activity was recorded for 60 min. Behavioral measures were calculated using the Activity Monitor program (Med Associates) and “center” was defined as an area beginning 10 cm from the walls. Animals were placed on an elevated plus maze apparatus for 6 min and their position was tracked using an overhead digital video camera and Anymaze software (Stoelting, Wood Dale, IL). The apparatus consisted of a plus shaped track with arms 18 cm long and 5 cm wide. Two arms were enclosed on 3 sides and the entire apparatus was elevated 50 cm above the bench top and surrounded by a white curtain under ambient room lighting. Mice were tested on an accelerating rotarod apparatus (Letica LE8200) over a period of 5 days. The first day consisted of 3 pre-training trials in which animals were habituated to the stationary apparatus 2 times for 3 min each, and then for an additional 3 min at 4 rpm. Animals that fell from the apparatus during these trials were returned to the rod until 3 min had elapsed. On subsequent days, animals were given 3 × 5 min trials per day with the rotation speed ramped from 4 to 20 rpm over the course of the trial. Animals that fell from the rod during these trials were returned to the rod once to discourage them from learning to fall as an escape strategy, however only the latency to the first fall was used for analysis. For all trails on all days, the intertrial interval was approximately 30 min during which animals were returned to their home cages. All fear conditioning trials were carried out in conditioning chambers placed in sound attenuating chambers (Med Associates, St Albans, VT) and freezing during each trial was monitored continuously using a video tracking and analysis system (Freezeframe, Actimetrix Software, Wilmette, IL). On day one animals were given a 3 min training trial consisting of 2 min of pre-exposure followed by a 2 s 0.6 mA continuous foot shock and 58 s of post-shock exposure. On subsequent days animals were reintroduced to the conditioning chamber in the absence of shock for 10 min. Percent of time spent freezing during the first 2 min of these trails was used to monitor extinction of the freezing response. The experiment was carried out in a circular pool 120 cm in diameter filled with water made opaque with white paint. A video tracking system (HVS Image VP-118) was used to record and analyze each animal’s behavior. A 10 cm square platform submerged 1 cm below the water surface was used as an escape platform and in the visible version of the task, a colored syringe barrel was affixed to the center to mark its location. For all phases of the experiment, 4 trials of no more than 120 s were given each day at 20 min inter-trial intervals. Animals that exceeded the 120 s limit were guided to the platform before being returned to their home cage. A single “sha**” trial in which animals were placed on the platform for 15 s, was performed before training on the first day of the visible, hidden and reversal phases of the task. Probe trials of 60 s, during which the platform was removed from the water maze, were performed at the end of training on days H5 and R5. In the visible platform phase of the task, the pool was surrounded by a curtain and the marked platform was placed in a different location on all four trials of a day. In the hidden and reversal phases, the curtain was removed to reveal the room cues and the hidden platform was placed in a fixed location for each phase. For all object recognition experiments, the apparatus consisted of an opaque plexiglass arena 71 cm square and 36 cm high. The arena was surrounded by a white curtain, and two prominent visual cues were affixed to two of the arena walls. Objects were glass, metal or ceramic items fixed in place with double stick tape (eg. padlock, glass chess piece, metal bottle cap, ceramic drawer knob). All objects, the arena and floor were cleaned with non-alcoholic antiseptic wipes between each trial. To habituate the animals to the testing environment, animals were handled in the testing room on the three days prior to testing. Animal movements were tracked and analyzed using Noldus Ethovision XT software and an overhead-mounted video camera. For the novel object version of the task animals were placed in the empty arena for 5 min first. Then three 5 min training trails were conducted in which three different objects were placed equidistantly in a diagonal line across the arena. Finally a 5 min. testing trial was conducted in which one of two peripheral objects was replaced with a new object. The delay between the empty arena trial and all of the training trials were 10 min, and the delay between the final training trial and the testing trial was 3 h. The spatial version of this task was carried out similarly except that instead of replacing one of the peripheral objects in the testing trail, it was moved to one of the two empty corners so that the three objects were now arranged in a “V”. Testing was performed essentially as described in97 and98. Animals were placed in startle chambers (SR-Lab startle response system, San Diego Instruments, San Diego, CA) containing a high-frequency speaker for generation of acoustic stimuli and a piezoelectric accelerometer to measure animal movements in response to stimulus presentations. A continuous level of 65 dB background white noise was presented through the experiment. The testing protocol consisted of 6 presentations of a 40 ms broadband 120 dB startle tone, followed by 10 pseudo-randomly interleaved presentations of background alone, prepulse trials consisting of stimuli at 3, 6 or 12 dB above background delivered 100 ms before the 40 ms broadband 120 dB startle tone, and non-prepulse trials in which the 120 dB startle tone was presented alone. This protocol was followed by another 6 presentations of the 120 dB startle tone alone. The interval between trials varied pseudorandomly between 15 and 30 s. PPI for each animal at a particular prepulse interval was calculated as the difference between the average startle magnitude on interspersed startle tone-alone trials and prepulse trials divided by the average startle magnitude on the same startle tone-alone trials. Mice were tested individually for spontaneous alternation during a single 6 min trial in a custom fabricated Y-maze apparatus. The maze consisted of three arms 40 cm in length, 17 cm high and 4 cm wide, separated by 120 degrees located on a table in the center of the testing room under dim lighting. Animals were placed at the end of one of the arms in a psedorandomly assigned manner. Movements were recorded by an overhead mounted video camera and monitored by an observer blinded to genotype. Arm entries were scored when all four of the animal’s paws entered an arm. The percent alternation was calculated as the number of triads containing entries into all three arms divided by the total number of triads (the total number of arms entered minus 2) × 100. Average number entries for all groups was 24.2 with a standard deviation of 5.5. Three RXRγ knockout and 2 wild-type mice with 13 or fewer arm entries were excluded from the analysis. Testing was conducted in the same apparatus used for open field behavior testing. Immediately before each 30 min trial, animals were given a single intraperitoneal injection of either 15 mg/kg cocaine or saline only. The testing protocol consisted of 3 days of habituation (1–3) in which all animals were administered saline only. Habituation was followed by 5 sensitization trials, conducted on days 4–8 and day 20, in which either cocaine or saline was administered as indicated. On day 22 all animals were tested again after receiving saline injections. Tests for statistical significance between groups were performed using Prism 7 (Graphpad Software, San Diego, CA, USA). Student’s unpaired, two-tailed t-tests were used for experiments involving 2 groups, and paired t-test for experiments in a single group comparing before to after drug application. 2-way ANOVA comparisons—with or without repeated measures as dictated by the experimental design—were used for analysis of all experiments involving multiple groups. Post-hoc comparisons for multiple group experiments were performed using Dunnett’s tests when comparisons were made to a single control group, and Tukey’s tests when comparisons were made among multiple groups. The results of these tests together with their associated P values are listed in the legends for their corresponding figure.Western blots
Q-PCR
Immunohistochemistry
Open field
Elevated plus maze
Accelerating rotarod
Fear conditioning
Morris water maze
Spatial and non-spatial object recognition
Prepulse inhibition
Y-maze spontaneous alternation
Behavioral sensitization to cocaine
Statistical analysis
References
Dawson, M. I. & **a, Z. The retinoid X receptors and their ligands. Biochim. Biophys. Acta 1821, 21–56 (2012).
Lefebvre, P., Benomar, Y. & Staels, B. Retinoid X receptors: Common heterodimerization partners with distinct functions. Trends Endocrinol. Metab. 21, 676–683 (2010).
Gilardi, F. & Desvergne, B. RXRs: Collegial partners. Subcell Biochem. 70, 75–102 (2014).
de Lera, A. R., Krezel, W. & Ruhl, R. An endogenous mammalian retinoid X Receptor ligand, at last!. ChemMedChem 11, 1027–1037 (2016).
Zhang, X. K. Targeting Nur77 translocation. Expert Opin. Ther. Targets 11, 69–79 (2007).
Unsworth, A. J. et al. RXR ligands negatively regulate thrombosis and hemostasis. Arterioscler. Thromb. Vasc. Biol. 37, 812–822 (2017).
Moraes, L. A. et al. Nongenomic signaling of the retinoid X receptor through binding and inhibiting Gq in human platelets. Blood 109, 3741–3744 (2007).
Zhou, H. et al. NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer Cell 17, 560–573 (2010).
Zhang, X. K. et al. Regulation of the nongenomic actions of retinoid X receptor-alpha by targeting the coregulator-binding sites. Acta Pharmacol. Sin. 36, 102–112 (2015).
Cramer, P. E. et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506 (2012).
Cummings, J. L. et al. Double-blind, placebo-controlled, proof-of-concept trial of bexarotene **n moderate Alzheimer’s disease. Alzheimers Res. Ther. 8, 4 (2016).
Ghosal, K. et al. A randomized controlled study to evaluate the effect of bexarotene on amyloid-beta and apolipoprotein E metabolism in healthy subjects. Alzheimers Dement (N Y) 2, 110–120 (2016).
Mariani, M. M. et al. Neuronally-directed effects of RXR activation in a mouse model of Alzheimer’s disease. Sci. Rep. 7, 42270 (2017).
Tesseur, I. & De Strooper, B. When the dust settles: What did we learn from the bexarotene discussion?. Alzheimers Res. Ther. 5, 54 (2013).
Tousi, B. The emerging role of bexarotene in the treatment of Alzheimer’s disease: current evidence. Neuropsychiatr. Dis. Treat. 11, 311–315 (2015).
McFarland, K. et al. Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson’s disease. ACS Chem. Neurosci. 4, 1430–1438 (2013).
Lerner, V., McCaffery, P. J. & Ritsner, M. S. Targeting retinoid receptors to treat schizophrenia: Rationale and progress to date. CNS Drugs 30, 269–280 (2016).
Chiang, M. Y. et al. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron 21, 1353–1361 (1998).
Huang, J. K. et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 14, 45–53 (2011).
Krzyzosiak, A. et al. Retinoid x receptor gamma control of affective behaviors involves dopaminergic signaling in mice. Neuron 66, 908–920 (2010).
Wietrzych-Schindler, M. et al. Retinoid x receptor gamma is implicated in docosahexaenoic acid modulation of despair behaviors and working memory in mice. Biol. Psychiatry 69, 788–794 (2011).
Wietrzych, M. et al. Working memory deficits in retinoid X receptor gamma-deficient mice. Learn. Mem. 12, 318–326 (2005).
Krezel, W. et al. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science 279, 863–867 (1998).
Golubeva, A. V., Moloney, R. D., O’Connor, R. M., Dinan, T. G. & Cryan, J. F. Metabotropic glutamate receptors in central nervous system diseases. Curr. Drug Targets 17, 538–616 (2016).
Yin, S. & Niswender, C. M. Progress toward advanced understanding of metabotropic glutamate receptors: Structure, signaling and therapeutic indications. Cell Signal 26, 2284–2297 (2014).
D’Antoni, S. et al. Dysregulation of group-I metabotropic glutamate (mGlu) receptor mediated signalling in disorders associated with intellectual disability and autism. Neurosci. Biobehav. Rev. 46(Pt 2), 228–241 (2014).
Luo, P., Li, X., Fei, Z. & Poon, W. Scaffold protein Homer 1: Implications for neurological diseases. Neurochem. Int. 61, 731–738 (2012).
Kalinowska, M. & Francesconi, A. Group I metabotropic glutamate receptor interacting proteins: Fine-tuning receptor functions in health and disease. Curr. Neuropharmacol. 14, 494–503 (2016).
Hu, J. H. et al. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron 68, 1128–1142 (2010).
Diering, G. H. et al. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515 (2017).
Luscher, C. & Huber, K. M. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459 (2010).
Kang, S. J. & Kaang, B. K. Metabotropic glutamate receptor dependent long-term depression in the cortex. Korean J. Physiol. Pharmacol. 20, 557–564 (2016).
Malenka, R. C. & Bear, M. F. LTP and LTD: An embarrassment of riches. Neuron 44, 5–21 (2004).
Collingridge, G. L., Peineau, S., Howland, J. G. & Wang, Y. T. Long-term depression in the CNS. Nat. Rev. Neurosci. 11, 459–473 (2010).
Dudek, S. M. & Bear, M. F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. U.S.A. 89, 4363–4367 (1992).
Mulkey, R. M. & Malenka, R. C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9, 967–975 (1992).
Volk, L. J., Daly, C. A. & Huber, K. M. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J. Neurophysiol. 95, 2427–2438 (2006).
Moult, P. R., Correa, S. A., Collingridge, G. L., Fitzjohn, S. M. & Bashir, Z. I. Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabotropic glutamate receptor-dependent long-term depression. J. Physiol. 586, 2499–2510 (2008).
Huber, K. M., Kayser, M. S. & Bear, M. F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257 (2000).
Kemp, N. & Bashir, Z. I. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology 38, 495–504 (1999).
Zhang, X. L., Upreti, C. & Stanton, P. K. Gbetagamma and the C terminus of SNAP-25 are necessary for long-term depression of transmitter release. PLoS ONE 6, e20500 (2011).
Bianchi, R., Chuang, S. C., Zhao, W., Young, S. R. & Wong, R. K. Cellular plasticity for group I mGluR-mediated epileptogenesis. J. Neurosci. 29, 3497–3507 (2009).
Jorntell, H. & Hansel, C. Synaptic memories upside down: Bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 52, 227–238 (2006).
Kano, M., Hashimoto, K. & Tabata, T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: A key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 2173–2186 (2008).
Conn, P. J., Battaglia, G., Marino, M. J. & Nicoletti, F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat. Rev. Neurosci. 6, 787–798 (2005).
Ribeiro, F. M. et al. Metabotropic glutamate receptor 5 knockout promotes motor and biochemical alterations in a mouse model of Huntington’s disease. Hum. Mol. Genet. 23, 2030–2042 (2014).
Aiba, A. et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell 79, 377–388 (1994).
Conquet, F. et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 372, 237–243 (1994).
Ichise, T. mGluR1 in Cerebellar purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science 288, 1832–1835 (2000).
Mukherjee, S. & Manahan-Vaughan, D. Role of metabotropic glutamate receptors in persistent forms of hippocampal plasticity and learning. Neuropharmacology 66, 65–81 (2013).
Lu, Y. M. et al. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J. Neurosci. 17, 5196–5205 (1997).
Xu, J., Zhu, Y., Contractor, A. & Heinemann, S. F. mGluR5 has a critical role in inhibitory learning. J. Neurosci. 29, 3676–3684 (2009).
Cohen, S. J. & Stackman, R. W. Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 285, 105–117 (2015).
Banks, P. J., Warburton, E. C., Brown, M. W. & Bashir, Z. I. Mechanisms of synaptic plasticity and recognition memory in the perirhinal cortex. Prog. Mol. Biol. Transl. Sci. 122, 193–209 (2014).
Ayoub, M. A. et al. Deleterious GRM1 mutations in schizophrenia. PLoS ONE 7, e32849 (2012).
Frank, R. A. et al. Clustered coding variants in the glutamate receptor complexes of individuals with schizophrenia and bipolar disorder. PLoS ONE 6, e19011 (2011).
Timms, A. E. et al. Support for the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA Psychiatry 70, 582–590 (2013).
Devon, R. S. et al. The genomic organisation of the metabotropic glutamate receptor subtype 5 gene, and its association with schizophrenia. Mol. Psychiatry 6, 311–314 (2001).
Ayalew, M. et al. Convergent functional genomics of schizophrenia: From comprehensive understanding to genetic risk prediction. Mol. Psychiatry 17, 887–905 (2012).
Matosin, N., Frank, E., Deng, C., Huang, X. F. & Newell, K. A. Metabotropic glutamate receptor 5 binding and protein expression in schizophrenia and following antipsychotic drug treatment. Schizophr. Res. 146, 170–176 (2013).
Gupta, D. S. et al. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse 57, 123–131 (2005).
Volk, D. W., Eggan, S. M. & Lewis, D. A. Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. Am. J. Psychiatry 167, 1489–1498 (2010).
Matosin, N., Fernandez-Enright, F., Lum, J. S. & Newell, K. A. Shifting towards a model of mGluR5 dysregulation in schizophrenia: Consequences for future schizophrenia treatment. Neuropharmacology 115, 73–91 (2017).
Maksymetz, J., Moran, S. P. & Conn, P. J. Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Mol Brain 10, 15 (2017).
Rook, J. M. et al. Biased mGlu5-positive allosteric modulators provide in vivo efficacy without potentiating mGlu5 modulation of NMDAR currents. Neuron 86, 1029–1040 (2015).
Braff, D. L., Geyer, M. A. & Swerdlow, N. R. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology 156, 234–258 (2001).
Swerdlow, N. R., Braff, D. L. & Geyer, M. A. Sensorimotor gating of the startle reflex: What we said 25 years ago, what has happened since then, and what comes next. J. Psychopharmacol. 30, 1072–1081 (2016).
Brody, S. A., Conquet, F. & Geyer, M. A. Disruption of prepulse inhibition in mice lacking mGluR1. Eur. J. Neurosci. 18, 3361–3366 (2003).
Kinney, G. G. et al. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J. Pharmacol. Exp. Ther. 306, 116–123 (2003).
Brody, S. A., Dulawa, S. C., Conquet, F. & Geyer, M. A. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol. Psychiatry 9, 35–41 (2004).
Brody, S. A., Conquet, F. & Geyer, M. A. Effect of antipsychotic treatment on the prepulse inhibition deficit of mGluR5 knockout mice. Psychopharmacology 172, 187–195 (2004).
Chen, H. H., Stoker, A. & Markou, A. The glutamatergic compounds sarcosine and N-acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology 209, 343–350 (2010).
Lipina, T., Weiss, K. & Roder, J. The ampakine CX546 restores the prepulse inhibition and latent inhibition deficits in mGluR5-deficient mice. Neuropsychopharmacology 32, 745–756 (2007).
Duncan, J. R. & Lawrence, A. J. The role of metabotropic glutamate receptors in addiction: Evidence from preclinical models. Pharmacol. Biochem. Behav. 100, 811–824 (2012).
Bourhis, E. et al. The transcription factors Nur77 and retinoid X receptors participate in amphetamine-induced locomotor activities. Psychopharmacology 202, 635–648 (2009).
Brtko, J. & Dvorak, Z. Role of retinoids, rexinoids and thyroid hormone in the expression of cytochrome p450 enzymes. Curr. Drug Metab. 12, 71–88 (2011).
Howard, L. A., Sellers, E. M. & Tyndale, R. F. The role of pharmacogenetically-variable cytochrome P450 enzymes in drug abuse and dependence. Pharmacogenomics 3, 185–199 (2002).
Wilkerson, J. R., Albanesi, J. P. & Huber, K. M. Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: Implications in health and disease. Semin. Cell Dev. Biol. 77, 51–62 (2018).
Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. U.S.A. 99, 7746–7750 (2002).
Bear, M. F., Huber, K. M. & Warren, S. T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004).
Dahlhaus, R. Of men and mice: Modeling the fragile X syndrome. Front. Mol. Neurosci. 11, 41 (2018).
Erickson, C. A. et al. Fragile X targeted pharmacotherapy: Lessons learned and future directions. J. Neurodev. Disord. 9, 7 (2017).
Zang, J. B. et al. A mouse model of the human Fragile X syndrome I304N mutation. PLoS Genet. 5, e1000758 (2009).
Kleppisch, T., Voigt, V., Allmann, R. & Offermanns, S. G(alpha)q-deficient mice lack metabotropic glutamate receptor-dependent long-term depression but show normal long-term potentiation in the hippocampal CA1 region. J. Neurosci. 21, 4943–4948 (2001).
Bhouri, M. et al. mGlu1 receptor-induced LTD of NMDA receptor transmission selectively at Schaffer collateral-CA1 synapses mediates metaplasticity. J. Neurosci. 34, 12223–12229 (2014).
Fitzjohn, S. M. et al. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci. Lett. 203, 211–213 (1996).
Harney, S. C., Rowan, M. & Anwyl, R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J. Neurosci. 26, 1128–1132 (2006).
Manahan-Vaughan, D. & Braunewell, K. H. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc. Natl. Acad. Sci. U.S.A. 96, 8739–8744 (1999).
Kemp, A. & Manahan-Vaughan, D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc. Natl. Acad. Sci. U.S.A. 101, 8192–8197 (2004).
Popkirov, S. G. & Manahan-Vaughan, D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb. Cortex 21, 501–509 (2011).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Irfan, M. et al. SNAP-25 isoforms differentially regulate synaptic transmission and long-term synaptic plasticity at central synapses. Sci. Rep. 9, 6403 (2019).
Bailey, C. P. et al. Galpha(i2) inhibition of adenylate cyclase regulates presynaptic activity and unmasks cGMP-dependent long-term depression at Schaffer collateral-CA1 hippocampal synapses. Learn. Mem. 15, 261–270 (2008).
Nicholls, R. E. et al. mGluR2 acts through inhibitory Galpha subunits to regulate transmission and long-term plasticity at hippocampal mossy fiber-CA3 synapses. Proc. Natl. Acad. Sci. U.S.A. 103, 6380–6385 (2006).
Zhang, X. L., Zhou, Z. Y., Winterer, J., Muller, W. & Stanton, P. K. NMDA-dependent, but not group I metabotropic glutamate receptor-dependent, long-term depression at Schaffer collateral-CA1 synapses is associated with long-term reduction of release from the rapidly recycling presynaptic vesicle pool. J. Neurosci. 26, 10270–10280 (2006).
Zhang, X. L., Sullivan, J. A., Moskal, J. R. & Stanton, P. K. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology 55, 1238–1250 (2008).
Geyer, M. A. & Swerdlow, N. R. Measurement of startle response, prepulse inhibition, and habituation. Curr. Protoc. Neurosci. https://doi.org/10.1002/0471142301.ns0807s03 (2001).
Valsamis, B. & Schmid, S. Habituation and prepulse inhibition of acoustic startle in rodents. J. Vis. Exp. 55, e3446 (2011).
Acknowledgements
This work was supported by Department of Defense Grant W81XWH-15-1-0404 (REN and PKS), and by a generous gift from the Broitman family (ERK). We thank Ronald M. Evans (Salk Institute) for the gift of RXRγ knockout mice.
Author information
Authors and Affiliations
Contributions
E.R.K., P.K.S. and R.E.N. conceived and designed the study. C.U., C.M.W., X-L.Z., M.J.Y., Z.Z., A.M.P., D.S.R. D.Y. and R.E.N. performed the experiments. C.U., X-L.Z. and R.E.N. analyzed the data and prepared the figures. R.E.N. and P.K.S. wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Upreti, C., Woodruff, C.M., Zhang, XL. et al. Loss of retinoid X receptor gamma subunit impairs group 1 mGluR mediated electrophysiological responses and group 1 mGluR dependent behaviors. Sci Rep 11, 5552 (2021). https://doi.org/10.1038/s41598-021-84943-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84943-x
- Springer Nature Limited