Abstract
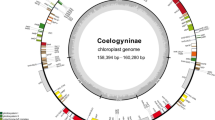
Allioideae includes economically important bulb crops such as garlic, onion, leeks, and some ornamental plants in Amaryllidaceae. Here, we reported the complete chloroplast genome (cpDNA) sequences of 17 species of Allioideae, five of Amaryllidoideae, and one of Agapanthoideae. These cpDNA sequences represent 80 protein-coding, 30 tRNA, and four rRNA genes, and range from 151,808 to 159,998 bp in length. Loss and pseudogenization of multiple genes (i.e., rps2, infA, and rpl22) appear to have occurred multiple times during the evolution of Alloideae. Additionally, eight mutation hotspots, including rps15-ycf1, rps16-trnQ-UUG, petG-trnW-CCA, psbA upstream, rpl32-trnL-UAG, ycf1, rpl22, matK, and ndhF, were identified in the studied Allium species. Additionally, we present the first phylogenomic analysis among the four tribes of Allioideae based on 74 cpDNA coding regions of 21 species of Allioideae, five species of Amaryllidoideae, one species of Agapanthoideae, and five species representing selected members of Asparagales. Our molecular phylogenomic results strongly support the monophyly of Allioideae, which is sister to Amaryllioideae. Within Allioideae, Tulbaghieae was sister to Gilliesieae-Leucocoryneae whereas Allieae was sister to the clade of Tulbaghieae- Gilliesieae-Leucocoryneae. Molecular dating analyses revealed the crown age of Allioideae in the Eocene (40.1 mya) followed by differentiation of Allieae in the early Miocene (21.3 mya). The split of Gilliesieae from Leucocoryneae was estimated at 16.5 mya. Biogeographic reconstruction suggests an African origin for Allioideae and subsequent spread to Eurasia during the middle Eocene. Cool and arid conditions during the late Eocene led to isolation between African and Eurasian species. African Allioideae may have diverged to South American taxa in the late Oligocene. Rather than vicariance, long-distance dispersal is the most likely explanation for intercontinental distribution of African and South American Allioideae species.
Similar content being viewed by others
Introduction
Allioideae Herbert, a subfamily of Amaryllidaceae (Asparagales), comprises four tribes, 13 genera and over 900 species1. The subfamily is widely distributed in temperate and subtropical regions of the Northern Hemisphere and South America, and occurs locally in South Africa2. Most Allioideae are economically important plants used in traditional medicine, horticulture, and also as ornamentals. Within Amaryllidaceae, Allioideae can easily be distinguished from the other subfamilies based on superior ovary and solid styles. These subfamilies are further characterized by possession of unique chemical compounds3. Molecular phylogenetic studies have demonstrated the monophyly of each subfamily of Amaryllidaceae using chloroplast (cp) DNA sequence data4,5,6. Despite the morphological, anatomical, chemical, and molecular distinctiveness of Allioideae, its sister group is controversial. Meerow et al.4 suggested that Allioideae is sister to the Agapanthoideae –Amaryllidoideae clade based on two cpDNA rbcL and trnL-F regions, which was also reported by Costa et al.7 inferred from four loci dataset. A more recent analysis of four cpDNA genes by Chen et al.6 found support for a sister relationship between Allioideae and Amaryllidoideae, which is in agreement with the results of Steele et al.5 and ** single nucleotide polymorphism markers for the identification of pineapple (Ananas comosus) germplasm. Hortic. Res. 2, 15056 (2015)." href="/article/10.1038/s41598-021-82692-5#ref-CR31" id="ref-link-section-d83091519e5398">31. In this study, different SSRs were identified among Allioideae that may be useful for studies of molecular markers and population genetics of Allium in particular and Allioideae in general (Tables S3 and S4). Furthermore, eight hotspot regions of cpDNA were identified, which can be used in future studies of interspecies relationships among Allium species (Table S2). Another study on the complete plastomes of Allium revealed different genes with high nucleotide diversity (including ndhK, ndhE, ndhA, rps16, psaI, rpl22, rpl32, and trnK-UUU) in comparison with the present study15. These various findings might be caused by different taxon sampling and an insufficient number of samples among the studies. However, these results provided preliminary data on nucleotide diversity of plastomes for further studies that include all Allium taxa to identify the common hotspot regions across Allium.
Phylogenetic relationships of Allioideae
Our MP and BI analyses consistently recovered Allioideae as sister to Amaryllidoideae (Fig. 2). This result is in line with previous molecular phylogenetic studies of Amaryllidaceae5,6. By contrast, Allioideae was found to be sister to a clade of Amaryllidoideae and Agapanthoideae inferred from data of nuclear ITS and plastid matK, ndhF, and rbcL7. Although Allioideae has superior ovary and solid style (vs. inferior ovary and hollow style in Amaryllidoideae), these characteristics are homoplasious in Asparagales32. Our phylogenomic study recovered Allieae as sister to the rest tribes of Allioideae (Fig. 2). The unique position of Allieae is also corroborated by having the synapomorphic, gynobasic style (vs. terminal in other tribes). Tulbaghieae, sister to Leucocoryneae-Gilliesieae, could be distinguished by the presence of corona in the flower. Moreover, the pseudogenization of cemA gene was only detected in Tulbaghieae. Gilliesieae and Leucocoryneae were strongly supported as sister in agreement with Sassone and Giussani2. This relationship is supported by several morphological characteristics such as terminal style position and absence of corona in the flower. In addition, both tribes were distributed in South America. In particular, Gilliesieae is restricted to Chile and Patagonia in Argentina, while Leucocoryneae is located in Argentina, Chile, Bolivia, Peru, Paraguay, Uruguay, and Brazil. Therefore, molecular phylogenetic relationships among tribes of Allioideae were supported by morphological and geographical evidence.
In the present study, Allium subg. Melanocrommyum and A. subg. Cyathophora were found to be non-monophyletic although 74 protein-coding genes were used (Fig. 2). Previous molecular phylogenetic studies of Allium revealed the non-monophyly of some subgenera53. Applying the Akaike information criterion, jModelTest v.2.1.754 assigned the GTR + I + Г model of molecular evolution to the combined dataset. Four MCMC chains were run simultaneously and sampled every 1000 generations for a total of 20 million generations. We plotted the log-likelihood scores of sample points against generation time using Tracer v.1.5; this ensured that stationarity was achieved after the first 2 million generations by determining whether the log-likelihood values of the sample points reached a stable equilibrium. In addition, we used the AWTY graphical system55 to compare split frequencies among runs and plot the cumulative split frequencies to ensure that stationarity was reached. The first 1000 (10%) sample trees from each run were discarded (representing burn-in), as determined using Tracer v.1.5. A maximum a posteriori tree was constructed by summarising the remaining trees from parallel runs into a majority-rule consensus tree, yielding posterior probability (PP) values for each clade.
Molecular dating analysis
To estimate the divergence times of tribes in Allioideae, we used BEAST v.1.856 based on 74 cpDNA coding regions. The BEAUti interface was used to generate input files for BEAST, in which the GTR + I + Г model, Yule speciation tree prior, and uncorrelated lognormal molecular clock model were applied. Two runs of 200 million generations were set for the MCMC chains, sampling every 1000 generations. Convergence of the stationary distribution was checked through visual inspection of the plotted posterior estimates using Tracer v.1.6. After discarding the first 20,000 (10%) trees as burn-in, the samples were summarised in a maximum clade credibility tree in TreeAnnotator v.1.6.1 using a PP limit of 0.50 and summarising the mean node heights. The mean and 95% HPD of each age estimate were obtained from the combined outputs using Tracer. The results were visualized using Figtree v.1.4.2 [http://tree.bio.ed.ac.uk/software/figtree/].
Age calibration was constrained to the phylogeny of Allioideae and its close relatives. The crown node (C1 in Fig. 3) of Yucca-Hosta was constrained with a uniform distribution from 20.7 to 37.5 mya following McKain et al.57, who estimated the divergence time of Agavoideae using 69 cpDNA coding genes. Three further calibration processes were implemented, as uniform distribution from 50.0 to 67.4 mya for the stem group of Amaryllidaceae (C2); from 42.0 to 61.7 mya for the crown group of Amaryllidaceae (C3); and from 38.1 to 56.5 mya for the stem node of Allioideae (C4).
Ancestral area reconstruction
Biogeographic data for species within Allioideae were compiled from their distributions described in the literature and herbarium specimens. The distribution range of Allioideae species and outgroups was divided into five areas: (A) Eurasia, (B) North America, (C) Africa, (D) South America, and (E) Australia. We coded each species based on the entire range of the species regardless of the sample’s biogeographic source. Ancestral area reconstruction and estimation of spatial patterns of geographic diversification within Allioideae were inferred using the BBM and S-DIVA as implemented in RASP v.2.1b (Reconstruct Ancestral State in Phylogenies, formerly S-DIVA)58. The BBM was run using the fixed state frequencies model (Jukes-Cantor) with equal among-site rate variations over two million generations, 10 chains each, and two parallel runs. In S-DIVA, the frequencies of ancestral ranges at a given node in ancestral reconstructions are averaged over all trees. For these analyses, we used all post burn-in trees obtained from BEAST analysis. The consensus tree used to map the ancestral distribution of each node was obtained using the Compute Condense option in RASP from stored trees. The maximum number of ancestral areas was set to five.
References
The Angiosperm Phylogeny Group et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 181, 1–20 (2016).
Sassone, A. B. & Giussani, L. M. Reconstructing the phylogenetic history of the tribe Leucocoryneae (Allioideae): reticulate evolution and diversification in South America. Mol. Phylogenet. Evol. 127, 437–448 (2018).
Kubitzki, K., Rohwer, J. G. & Bittrich, V. The Families and Genera of Vascular Plants (Springer, Berlin, 1990).
Meerow, A. W. et al. Systematics of Amaryllidaceae based on cladistic analysis of plastid sequence data. Am. J. Bot. 86, 1325–1345 (1999).
Steele, P. R. et al. Quality and quantity of data recovered from massively parallel sequencing: Examples in Asparagales and Poaceae. Am. J. Bot. 99, 330–348 (2012).
Chen, S., Kim, D.-K., Chase, M. W. & Kim, J.-H. Networks in a large-scale phylogenetic analysis: reconstructing evolutionary history of Asparagales (Lilianae) based on four plastid genes. PLoS ONE 8, e59472 (2013).
Costa, L. et al. Divide to conquer: evolutionary history of Allioideae Tribes (Amaryllidaceae) is linked to distinct trends of karyotype evolution. Front. Plant Sci. 11, 1–15 (2020).
**e, D. F. et al. Insights into phylogeny, age and evolution of Allium (Amaryllidaceae) based on the whole plastome sequences. Ann. Bot. 125, 1039–1055 (2020).
Nguyen, N. H., Driscoll, H. E. & Specht, C. D. A molecular phylogeny of the wild onions (Allium; Alliaceae) with a focus on the western North American center of diversity. Mol. Phylogenet. Evol. 47, 1157–1172 (2008).
Friesen, N., Fritsch, R. & Blattner, F. Phylogeny and new intrageneric classification of Allium (Alliaceae) based on nuclear ribosomal DNA ITS sequences. Aliso 22, 372–395 (2006).
Souza, G., Crosa, O., Speranza, P. & Guerra, M. Phylogenetic relations in tribe Leucocoryneae (Amaryllidaceae, Allioideae) and the validation of Zoellnerallium based on DNA sequences and cytomolecular data. Bot. J. Linn. Soc. 182, 811–824 (2016).
Cox, C. B., Moore, P. D. & Ladle, R. Biogeography: An Ecological and Evolutionary Approach (Wiley-Blackwell, New York, 2016).
Kim, C., Kim, S.-C. & Kim, J.-H. Historical biogeography of Melanthiaceae: a case of out-of-North America through the bering land bridge. Front. Plant Sci. 10, 396 (2019).
Morley, R. J. Interplate dispersal paths for megathermal angiosperms. Perspect. Plant Ecol. Evol. Syst. 6, 5–20 (2003).
Nie, Z.-L. et al. Evolution of the intercontinental disjunctions in six continents in the Ampelopsis clade of the grape family (Vitaceae). BMC Evol. Biol. 12, 17 (2012).
McLoughlin, S. The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Aust. J. Bot. 49, 271 (2001).
Li, Q.-Q., Zhou, S.-D., Huang, D.-Q., He, X.-J. & Wei, X.-Q. Molecular phylogeny, divergence time estimates and historical biogeography within one of the world’s largest monocot genera. AoB Plants 8, plw41 (2016).
Choi, J. W. et al. Organelle inheritance and genome architecture variation in isogamous brown algae. Sci. Rep. 10, 2048 (2020).
Crosby, K. & Smith, D. R. Does the mode of plastid inheritance influence plastid genome architecture?. PLoS ONE 7, e46260 (2012).
Givnish, T. J. et al. Orchid historical biogeography, diversification, Antarctica and the paradox of orchid dispersal. J. Biogeogr. 43, 1905–1916 (2016).
**e, D.-F. et al. Phylogeny of Chinese Allium species in section Daghestanica and adaptive evolution of Allium (Amaryllidaceae, Allioideae) species revealed by the chloroplast complete genome. Front. Plant Sci. https://doi.org/10.3389/fpls.2019.00460 (2019).
Huo, Y. et al. Complete chloroplast genome sequences of four Allium species: comparative and phylogenetic analyses. Sci. Rep. 9, 12250 (2019).
**ao-Ming, Z. et al. Inferring the evolutionary mechanism of the chloroplast genome size by comparing whole-chloroplast genome sequences in seed plants. Sci. Rep. 7, 1555 (2017).
Smith, D. R. Unparalleled GC content in the plastid DNA of Selaginella. Plant Mol. Biol. 71, 627–639 (2009).
Ravi, V., Khurana, J. P., Tyagi, A. K. & Khurana, P. An update on chloroplast genomes. Plant Syst. Evol. 271, 101–122 (2008).
Do, H. D. K., Kim, C., Chase, M. W. & Kim, J. Implications of plastome evolution in the true lilies (monocot order Liliales). Mol. Phylogenet. Evol. 148, 106818 (2020).
Millen, R. S. et al. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell 13, 645–658 (2001).
Wang, R.-J. et al. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evol. Biol. 8, 36 (2008).
Dong, W.-L. et al. Molecular evolution of chloroplast genomes of orchid species: insights into phylogenetic relationship and adaptive evolution. Int. J. Mol. Sci. 19, 716 (2018).
Wang, X. et al. The USDA cucumber (Cucumis sativus L.) collection: genetic diversity, population structure, genome-wide association studies, and core collection development. Hortic. Res. 5, 64 (2018).
Zhou, L. et al. Develo** single nucleotide polymorphism markers for the identification of pineapple (Ananas comosus) germplasm. Hortic. Res. 2, 15056 (2015).
Pires, C. et al. Phylogeny, genome size, and chromosome evolution of Asparagales. Aliso 22, 287–304 (2006).
Li, Q.-Q. et al. Phylogeny and biogeography of Allium (Amaryllidaceae: Allieae) based on nuclear ribosomal internal transcribed spacer and chloroplast rps16 sequences, focusing on the inclusion of species endemic to China. Ann. Bot. 106, 709–733 (2010).
Peruzzi, L., Carta, A. & Altinordu, F. Chromosome diversity and evolution in Allium (Allioideae, Amaryllidaceae). Plant Biosyst. Int. J. Deal Asp. Plant Biol. 151, 212–220 (2017).
Smirnov, S., Skaptsov, M., Shmakov, A., Fritsch, R. M. & Friesen, N. Spontaneous hybridization among Allium tulipifolium and A. robustum (Allium subg. Melanocrommyum, Amaryllidaceae) under cultivation. Phytotaxa 303, 155 (2017).
Pigg, K. B., Bryan, F. A. & DeVore, M. L. Paleoallium billgenseli gen. et sp. nov.: Fossil Monocot Remains from the Latest Early Eocene Republic Flora, Northeastern Washington State, USA. Int. J. Plant Sci. 179, 477–486 (2018).
Dubouzet, J. G. & Shinoda, K. Relationships among Old and New World Alliums according to ITS DNA sequence analysis. Theor. Appl. Genet. 98, 422–433 (1999).
Zachos, J. Trends, rhythms, and aberrations in global climate 65 Ma to Present. Science (80-) 292, 686–693 (2001).
Désamoré, A. et al. Out of Africa: north-westwards Pleistocene expansions of the heather Erica arborea. J. Biogeogr. 38, 164–176 (2011).
Antunes Carvalho, F. & Renner, S. S. A dated phylogeny of the papaya family (Caricaceae) reveals the crop’s closest relatives and the family’s biogeographic history. Mol. Phylogenet. Evol. 65, 46–53 (2012).
Müller, S. et al. Intercontinental long-distance dispersal of Canellaceae from the New to the Old World revealed by a nuclear single copy gene and chloroplast loci. Mol. Phylogenet. Evol. 84, 205–219 (2015).
Givnish, T. J. et al. Phylogenomics and historical biogeography of the monocot order Liliales: out of Australia and through Antarctica. Cladistics 32, 581–605 (2016).
Doyle, J. J. & Doyle, J. L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987).
Kearse, M. et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Dierckxsens, N., Mardulyn, P. & Smits, G. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. https://doi.org/10.1093/nar/gkw955 (2016).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115–e115 (2012).
Schattner, P., Brooks, A. N. & Lowe, T. M. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33, W686–W689 (2005).
Greiner, S., Lehwark, P. & Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 47, W59–W64 (2019).
Librado, P. & Rozas, J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Kurtz, S. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 29, 4633–4642 (2001).
Katoh, K. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Cummings, M. P. PAUP* [Phylogenetic Analysis Using Parsimony (and Other Methods)]. in Dictionary of Bioinformatics and Computational Biology (Wiley, 2004). doi:https://doi.org/10.1002/0471650129.dob0522.
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772–772 (2012).
Nylander, J. A. A., Olsson, U., Alström, P. & Sanmartín, I. Accounting for phylogenetic uncertainty in biogeography: a Bayesian approach to dispersal-vicariance analysis of the thrushes (Aves: Turdus). Syst. Biol. 57, 257–268 (2008).
Drummond, A. J. & Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (2007).
McKain, M. R. et al. A phylogenomic assessment of ancient polyploidy and genome evolution across the Poales. Genome Biol. Evol. https://doi.org/10.1093/gbe/evw060 (2016).
Yu, Y., Harris, A. J., Blair, C. & He, X. RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Mol. Phylogenet. Evol. 87, 46–49 (2015).
Acknowledgments
This work was supported by the Scientific Research (KNA 1-1-13, 14-1) of Korea National Arboretum and the National Research Foundation (NRF-2017R1D1A1B06029326).
Author information
Authors and Affiliations
Contributions
J.-H.K. and H.J.C. conceived the experiments; J.N., C.K., and H.D.K.D. conducted the experiments, analyzed the data, and wrote the draft manuscript; J.-H.K. and H.J.C. revised the draft manuscript. All authors agreed to the final form of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Namgung, J., Do, H.D.K., Kim, C. et al. Complete chloroplast genomes shed light on phylogenetic relationships, divergence time, and biogeography of Allioideae (Amaryllidaceae). Sci Rep 11, 3262 (2021). https://doi.org/10.1038/s41598-021-82692-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82692-5
- Springer Nature Limited