Abstract
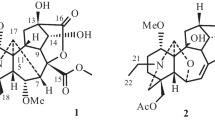
Five sets of germacrane isomers (1/8/17, 2/7/10/11/13/16/18, 3/4/5/14/20, 6/12/15, and 9/19) with different skeletal types, including seven new ones (1–3, 8–9, and 15–16) were isolated from the whole plant of Carpesium divaricatum. Among them, there are six pairs of stereoisomers (1/8, 2/13, 4/14, 6/12, 7/11 and 10/11). The planar structures and relative configurations of the new compounds were elucidated by detailed spectroscopic analysis. The absolute configurations of 4, 10, 11, and 17 were established by circular dichroism (CD) spectra and X-ray crystallographic analyses, and the stereochemistry of the new compounds 1–3, 8–9, and 15–16 were determined by similar CD spectra with 4, 10, 11, and 17, respectively. The confusion in the literature about subtypes I and II of germacranolides was clarified in this paper. The NMR data of 10–11, and the absolute configurations of the known compounds 4–6, 13–14, and 17–20 were reported for the first time. Compounds 13, 17, and 18 showed cytotoxicity against human cervical (HeLa), colon (LoVo) and stomach cancer (BGC-823) cell lines with IC50 values in the range 4.72–13.68 μM compared with the control cis-platin (7.90–15.34 μM).
Similar content being viewed by others
Introduction
The genus Carpesium (Asteraceae) includes 25 species worldwide, most of which are distributed across Asia and Europe, particularly in southwest China1,2. The plant Carpesium divaricatum, as a Chinese folk medicine, has been used for the treatment of fevers, colds, bruises, and inflammatory diseases3,4,5,6,7. Previous investigations indicated that a series of diverse compounds were isolated, including sesquiterpenoid lactones, acyclic diterpenes, and thymol derivatives, with the sesquiterpenoid lactones being the major constituents5,6,7,8,9,10,Plant Material The whole plants of C. divaricatum were collected from EnShi, Hubei province of China, in August of 2013. They were identified by Prof. Ben-Gang Zhang of Institute of Medicinal Plant Development. A voucher specimen (No. 20130828) was deposited in the National Compound Library of Traditional Chinese Medicines, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS & PUMC), China. The air-dried plants (9 kg) were extracted three times (7 days each time) with EtOH–H2O (95:5) at room temperature. The combined extract was concentrated under reduced pressure to furnish a dark brown residue (570 g), which was suspended in H2O and partitioned in turn with petroleum ether (bp 60–90 °C), EtOAc, and n-BuOH. The EtOAc extract (207 g) was separated chromatographically on silica gel column (60–100 mesh, 16 × 20 cm) with a gradient mixture of CH2Cl2–MeOH (100:1, 60:1, 30:1, 15:1, and 6:1) as eluent. Five fractions were collected according to TLC analysis. Fraction A (CH2Cl2–MeOH, 100:1, 140 g) was separated by silica gel column chromatography (CC) (100–200 mesh, 16 × 20 cm) with petroleum ether–Aceton (50:1, 25:1, 20:1, 15:1, 12:1, 10:1, 7:1, 5:1, 3:1, and 1:1) as eluent to give fractions A1–A11. Fraction A10 (petroleum ether–Aceton, 3:1, 40 g) was separated by Sephadex LH-20 CC (5 × 200 cm, MeOH) to give Fr.A10S1–Fr.A10S3. Fraction A10S2 (20 g) was then subjected to MCI gel CC (6 × 50 cm) with a gradient mixture of MeOH–H2O (60:40, 80:20, and 100:0, 4000 mL each) to give three fractions (Fr.A10S2M1–Fr.A10S2M3). Fraction A10S2M2 (MeOH–H2O, 80:20, 3 g) was purified using preparative HPLC (Daisogel–C18–100 A, 10μm; 250 × 30 mm; 20 mL/min, 60% MeOH in H2O) to yield 1 (30 mg). Fraction A10S2M2 (13 g) was further separated chromatographically on silica gel column (200–300 mesh, 5 × 50 cm) with a gradient mixture of CH2Cl2– MeOH (150:1, 100:1, 50:1, and 20:1) as eluent, and a total of 86 fractions (Fr.A10S2M2-1–86, 200 mL each) were collected. Fraction A10S2M2-56–60 were recrysted with CH2Cl2–MeOH (10:1) to yield 17 (200 mg). Fraction A10S2M2-70 (100 mg) was purified using semipreparative HPLC (YMC–Pack ODS–A column; 5μm; 250 × 10 mm; 2 mL/min, 50% MeOH in H2O) to yield 13 (20 mg) and 14 (30 mg). Fraction A10S2M2-69 (100 mg) was purified using semipreparative HPLC with MeOH–H2O (50:50) to yield 10 (10 mg), 11 (9 mg), and a mixture of 9 and 12 (25 mg). The mixture of 9 and 12 (25 mg) was further purified using semipreparative HPLC (40–80% MeCN in H2O for 40 min) to yield 9 (4.5 mg) and 12 (6.2 mg). Fraction A10S2M2-15–19 (140 mg) were purified using semipreparative HPLC (40–60% MeOH in H2O for 20 min, and followed by 60–80% for 20 min) to yield 6 (10 mg) and 7 (50 mg). Fraction A10S2M2-20–24 (2 g) were separated by preparative HPLC (65% MeOH in H2O) and semipreparative HPLC (60% MeOH in H2O for 10 min, and followed by 60–90% for 25 min; 40–85% MeCN in H2O for 40 min) to yield 5 (6.8 mg), 3 (4 mg), 15 (5 mg), and 18 (12 mg). Fraction A10S2M2-34–50 (1.5 g) were separated by preparative HPLC (70% MeOH in H2O) and semipreparative HPLC (52–75% MeOH in H2O for 25 min, and followed by 75–95% for 10 min) to yield 4 (50 mg). Fraction A10S2M2-74–79 (140 mg) were purified using semipreparative HPLC (60–80% MeOH in H2O for 25 min, and followed by 80–90% for 20 min; 30–70% MeCN in H2O for 40 min) and to yield 8 (4 mg) and 19 (35 mg). Fraction A9 (petroleum ether-Aceton, 5:1, 30 g) was separated by Sephadex LH-20 CC (5 × 200 cm, MeOH) to give Fr.A9S1–Fr.A9S3. Fraction A9S2 (20 g) was then subjected to MCI gel CC (6 × 50 cm) with a gradient mixture of MeOH–H2O (60:40, 80:20, and 100:0, 4000 mL each) to give three fractions (Fr.A9S2M1–Fr.A9S2M3). Fraction A9S2M2 (10 g) was further separated chromatographically on silica gel column (100–200 mesh, 5 × 50 cm)with a gradient mixture of petroleum ether–Aceton (10:1, 7:1, 5:1, 3.5:1, 2:1, and 1:1) as eluent, and a total of 200 fractions (Fr.A9S2M2-1–200, 50 mL each) were collected. Fraction A9S2M2-113–123 (1 g) were separated by preparative HPLC (65% MeOH in H2O) and semipreparative HPLC (68% MeOH in H2O for 50 min; 40–80% MeCN in H2O for 40 min) to yield 2 (4.6 mg). Fraction A9S2M2-107–112 (2.5 g) were separated by silica gel column chromatography (CC) (200–300 mesh, 5 × 40 cm) with CH2Cl2–MeOH (150:1, 75:1, 30:1, and 15:1) as eluent to give Fr. A9S2M2-107-112-A1–Fr. A9S2M2-107-112-A8. Fraction A9S2M2-107–112–A3 (CH2Cl2–MeOH, 75: 1, 500 mg) was further purified using semipreparative HPLC(65–90% MeOH in H2O for 40 min; 40–80% MeCN in H2O for 40 min) to yield 16 (10 mg) and 20 (10 mg). X-ray diffraction data were collected on the Agilent GEMINITME instrument (CrysAlisPro software, Version 1.171.35.11), with enhanced Cu Kα radiation (λ = 1.54184 Å). The structure was solved by direct methods and refined by full-matrix least-squares techniques (SHELXL-97). All non-hydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atoms were located by geometrical calculations and from positions in the electron density maps. Crystallographic data (excluding structure factors) for 4, 10, 11, and 17 in this paper have been deposited with the Cambridge Crystallographic Data Centre (deposition numbers CCDC 1407813, 1407814, and 1407812). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 12 23336033 or e-mail: deposit@ccdc.cam.ac.uk). A colorless orthorhombic crystal (0.58 × 0.48 × 0.45 mm) of 4 was obtained from MeOH. Crystal data were C24H34O9, M = 466.51, T = 99.5 K, orthorhombic, space group P212121, a = 9.05085(16) Å, b = 14.4036(3) Å, c = 19.1015(3) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 2490.16(8) Å3, Z = 4, ρ = 1.244 mg/mm3, μ(Cu Kα) = 0.790 mm−1, measured reflections = 8581, unique reflections = 4706 (Rint = 0.0189), largest difference peak/hole = 0.247/ −0.197 e Å−3, and flack parameter = −0.10(12). The final R indexes [I > 2σ (I)] were R1 = 0.0338, and wR2 = 0.0884. The final R indexes (all data) were R1 = 0.0345, and wR2 = 0.0890. The goodness of fit on F2 was 1.055. After trying several solvent systems, the mixture trigonal crystal (0.50 × 0.20 × 0.20 mm) of 10 and 11 (1:1) was from MeOH. Main parameters: C25.487355H27O10.25, M = 497.32, T = 103.1 K, trigonal, space group P3221, a = 18.3947(4) Å, b = 18.3947(4) Å, c = 28.7027(5) Å, α = 90.00°, β = 90.00°, γ = 120.00°, V = 8410.8(3) Å3, Z = 12, ρ = 1.178 mg/mm3, μ(Cu Kα) = 0.774 mm−1, measured reflections = 56790, unique reflections = 10785 (Rint = 0.0359), largest difference peak/hole = 0.857/−0.560 e Å−3, and flack parameter = −0.0(2). The final R indexes [I > 2σ (I)] were R1 = 0.0668, and wR2 = 0.1781. The final R indexes (all data) were R1 = 0.0737, and wR2 = 0.1857. The goodness of fit on F2 was 1.027. A colorless monoclinic crystal (0.55 × 0.40 × 0.36 mm) of 17 was grown from CH2Cl2-MeOH (20:1). Crystal data: C23H34O9, M = 454.50, T = 101.0 K, monoclinic, space group P21, a = 13.5135(4) Å, b = 9.5039(2) Å, c = 18.8280(6) Å, α = 90.00°, β = 105.051(3)°, γ = 90.00°, V = 2335.13(11) Å3, Z = 4, ρ = 1.293 mg/mm3, μ(Cu Kα) = 0.827 mm−1, measured reflections = 16917, unique reflections = 8859 (Rint = 0.0244), largest difference peak/hole = 0.759/−0.445 e Å−3, and flack parameter = −0.00(11). The final R indexes [I > 2σ (I)] were R1 = 0.0390, and wR2 = 0.1006. The final R indexes (all data) were R1 = 0.0397, and wR2 = 0.1012. The goodness of fit on F2 was 1.024. The assay was run in triplicate. In a 96-well plate, each well was plated with 2 × 104 cells. After cell attachment overnight, the medium was removed, and each well was treated with 100 μL of medium containing 0.1% DMSO or different concentrations of the test compounds and the positive control cis-platin. The plate was incubated for 4 days at 37 °C in a humidified, 5% CO2 atmosphere. Cytotoxicity was determined using a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay29. After addition of 10 μL MTT solution (5 mg/mL), cells were incubated at 37 °C for 4 h. After adding 150 μL DMSO, cells were shaken to mix thoroughly. The absorbance of each well was measured at 540 nm in a Multiscan photometer. The IC50 values were calculated by Origin software and listed in Table 3.Extraction and Isolation
X-ray Crystal Structure Analysis
Cytotoxicity Assays
References
Mabberley, D. J. Mabberley’s Plant Book (3.ed), 154 (Cambridge University Press: Cambridge, 2008).
Zhang, J. P. et al. The genus carpesium: a review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 163, 173–191 (2015).
Editorial board of Chinese Materia Medica. Chinese Materia Medica, Vol. 21 (eds Hu, X. M. et al.) 7761 (Shanghai Science & Technology Press, Shanghai, 1999).
Kim, E. J. et al. Suppression by a sesquiterpene lactone from Carpesium divaricatum of inducible nitric oxide synthase by inhibiting nuclear factor-κB activation. Biochem. Pharmacol. 61, 903–910 (2001).
Zee, O. P. et al. A new cytotoxic acyclic diterpene from Carpesium divaricatum. Arch. Pharm. Res. 22, 225–227 (1999).
Zee, O. P., Kim, D. K. & Lee, K. R. Thymol derivatives from Carpesium divaricatum. Arch. Pharm. Res. 21, 618–620 (1998).
Chung, I. M. et al. Antiplasmodial activity of isolated compounds from Carpesium divaricatum. Phytother. Res. 24, 451–453 (2010).
Maruyama, M. Sesquiterpene lactones from Carpesium divaricatum. Phytochemistry. 29, 547–550 (1990).
Kim, D. K., Lee, K. R. & Zee, O. P. Sesquiterpene lactones from Carpesium divaricatum. Phytochemistry. 46, 1245–1247 (1997).
Kim, D. K. et al. Four new cytotoxic germacranolides from Carpesium divaricatum. J. Nat. Prod. 60, 1199–1202 (1997).
**e, W. D. et al. Sesquiterpenoids from Carpesium divaricatum and their cytotoxic activity. Fitoterapia. 83, 1351–1355 (2012).
Zhang, T. et al. New highly oxygenated germacranolides from Carpesium divaricatum and their cytotoxic activity. Sci.Rep. 6, 27237, https://doi.org/10.1038/srep27237 (2016).
Moon, H. I. Antiplasmodial activity of ineupatorolides A from Carpesium rosulatum. Parasitol Res. 100, 1147–1149 (2007).
Liu, Q. X. et al. Isolation, structure elucidation, and absolute configuration of highly oxygenated germacranolides from Carpesium cernuum. J. Nat. Prod. 10, 2479–2486 (2016).
Baruah, R. N., Sharma, R. P. & Thyagarajan, G. Unusual germacranolides from Inula eupatorioides. J. Org. Chem. 45, 4838–4843 (1980).
Baruah, N. C. et al. Germacranolides of Inula eupatorioides. 2. Absolute configuration of the ineupatorolides. J. Org. Chem. 47, 137–140 (1982).
Goswami, A. C. et al. Germacranolides from Inula cappa. Phytochemistry. 23, 367–372 (1984).
Lee, H. J. et al. A germacranolide sesquiterpene lactone suppressed inducible nitric oxide synthase by downregulating NF-κB activity. Can. J. Physiol. Pharmacol. 89, 232–237 (2011).
Moon, H. I. & Zee, O. Antiproliferative effect from sesquiterpene lactones of Carpesium rosulatum MlQ consumed in South Korea on the five human cancer cell Lines. Rec. Nat. Prod. 4, 3149–3155 (2010).
Moon, H. I. & Zee, O. Sesquiterpene lactones from Carpesium rosulatum with potential cytotoxicity against five human cancer cell lines. Hum. Exp. Toxicol. 30, 1083–1087 (2010).
Wang, F. Y. et al. Sesquiterpene lactones from Inula cappa. Phytochem. Lett. 5, 639–642 (2012).
Gao, X., Lin, C. J. & Jia, Z. J. Cytotoxic germacranolides and acyclic diterpenoides from the seeds of Carpesium triste. J. Nat. Prod. 70, 830–834 (2007).
Bohlmann, F., Singh, P. & Jakupovic, J. Further ineupatorolide-like germacranolides from Inula cuspidata. Phytochemistry. 21, 157–160 (1982).
Lin, Y. L. & Ou, J. C. Napalolides A–D, four new sesquiterpene lactones from Carpesium nepalense. J. Nat. Prod. 59, 991–993 (1996).
Kim, M. R. et al. Sesquiterpene lactones from Carpesium triste var. manshuricum. Phytochemistry. 52, 113–115 (1999).
Kim, M. R. et al. Cytotoxic germacranolide sesquiterpene lactones from Carpesium triste var. manshuricum. Arch. Pharm. Res. 30, 556–560 (2007).
Liang, Q. L., Jiang, J. H. & Min, Z. D. A germacrane sesquiterpenoid from Vernonia patula. Chin. J. Nat. Med. 8, 104–106 (2010).
Gonzalez, A. G. et al. Germacranolides from Allagopappus viscosissimus. Phytochemistry. 31, 330–331 (1992).
Wang, B. J. et al. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food Chem. Toxicol. 43, 543–552 (2005).
Acknowledgements
This work was financially supported by the CAMS Innovation Fund for Medical Sciences (CIFMS, 2016-I2M-1-010), the Chinese National S&T Special Project on Major New Drug Innovation (2017ZX09301059), and the National Key Research and Development Program of China (2017YFD0201400-2).
Author information
Authors and Affiliations
Contributions
Zhong-Mei Zou designed the study; Tao Zhang performed the experiments with the help of Jia-Huan Chen, **-Guang Si, Gang Ding, Qiu-Bo Zhang, Hong-Wu Zhang, and Hong-Mei Jia. The manuscript was prepared by Tao Zhang, and Zhong-Mei Zou. All authors discussed the results and their interpretation and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, T., Chen, JH., Si, JG. et al. Isolation, Structure Elucidation, and Absolute Configuration of Germacrane Isomers from Carpesium divaricatum. Sci Rep 8, 12418 (2018). https://doi.org/10.1038/s41598-018-30782-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30782-2
- Springer Nature Limited
This article is cited by
-
Isolation of Nannocystis species from Iran and exploring their natural products
Archives of Microbiology (2022)