Abstract
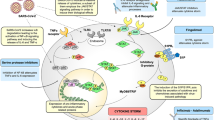
The rapid development of safe and effective vaccines helped to prevent severe disease courses after SARS-CoV-2 infection and to mitigate the progression of the COVID-19 pandemic. While there is evidence that vaccination may reduce the risk of develo** post-COVID-19 conditions (PCC), this effect may depend on the viral variant. Therapeutic effects of post-infection vaccination have been discussed but the data for individuals with PCC remains inconclusive. In addition, extremely rare side effects after SARS-CoV-2 vaccination may resemble the heterogeneous PCC phenotype. Here, we analyze the plasma levels of 25 cytokines and SARS-CoV-2 directed antibodies in 540 individuals with or without PCC relative to one or two mRNA-based COVID-19 vaccinations as well as in 20 uninfected individuals one month after their initial mRNA-based COVID-19 vaccination. While none of the SARS-CoV-2 naïve individuals reported any persisting sequelae or exhibited PCC-like dysregulation of plasma cytokines, we detected lower levels of IL-1β and IL-18 in patients with ongoing PCC who received one or two vaccinations at a median of six months after infection as compared to unvaccinated PCC patients. This reduction correlated with less frequent reporting of persisting gastrointestinal symptoms. These data suggest that post-infection vaccination in patients with PCC might be beneficial in a subgroup of individuals displaying gastrointestinal symptoms.
Similar content being viewed by others
Introduction
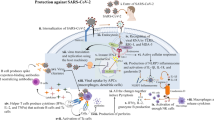
Infection with the zoonotic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes the coronavirus disease 2019 (COVID-19)1,2. Due to the multi-organ tropism of SARS-CoV-2, COVID-19 manifestations are often systemic and characterized by a broad severity spectrum with high morbidity and an elevated risk of mortality in distinct patient groups1,3. After the global spread of SARS-CoV-2 in 2020, the rapid development of several novel vaccine platforms within one year was key to mitigate the pandemic4,5,6,7,8. This unprecedented achievement was possible due to prior knowledge from the development and preclinical studies of vaccine candidates against SARS-CoV and Middle Eastern respiratory syndrome coronavirus (MERS-CoV) that identified the spike protein of human coronaviruses as the cardinal antigenic target to generate broad neutralizing B and T cell responses4,9. In August 2023, the two licensed mRNA-based COVID-19 vaccines BNT162b2 (BioNTech/Pfizer)10 and mRNA-1273 (Moderna)11, both of which encode a modified full-length SARS-CoV-2 S1 spike protein designed to stabilize the prefusion conformation, account for 90% of administered doses in the European Union and the United States12. Large clinical trials and real-world data clearly show that both vaccines are extremely safe and provide high protection against symptomatic and severe infection by eliciting neutralizing B and T cell responses including immunological memory that are also effective against different emerging variants of concern in single-dose or (heterologous) booster settings13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28. Notably, COVID-19 vaccination has similar risk and safety profiles in immunocompromised individuals29, patients with cancer30,31,32,33 or during pregnancy34.
Although vaccines are highly successful in reducing morbidity and mortality of acute COVID-19, their efficacy in preventing long-term consequences of a SARS-CoV-2 infection is less clear. Around 10-15% of COVID-19 patients have persisting health impairments beyond four weeks of symptom onset that are heterologous in their expression and can last for months with significant impairments for the quality of life35,36,37,38,39. For earlier variants of SARS-CoV-2 studies suggested that the likelihood of develo** symptoms after infection (post-COVID-19 condition; PCC) is less frequent in individuals with pre-infection vaccination compared to those without40,92. Nevertheless, this requires further investigation given the lack of longitudinal symptom reporting or plasma sampling in our study.
Finally, it is important to state that the data presented in this manuscript does not provide any hints that post-infection vaccination might exacerbate PCC-like symptoms or adversely impact individuals with earlier infection. We also did not detect any PCC-like signature in uninfected individuals after initial mRNA-based vaccination, neither did we notice any general imprint that could be interpreted as priming for potential PCC development after booster vaccination. Our data suggests that vaccination in PCC patients may play a role in ameliorating post-infection gut-related symptoms via IL-1 family cytokines. While we did not observe any vaccination-associated adverse events, these can affect a very small number of vaccinees and require larger studies. It is nevertheless important to acknowledge virus-induced and vaccine-induced pathology as distinct entities to understand their underpinnings and develop targeted treatments.
Together, our study corroborates the safety and efficiency of mRNA-based COVID-19 vaccines in SARS-CoV-2 naïve individuals or after infection. In addition, we provide biomarker-based data that suggests a benefit of post-infection vaccination for patients with PCC who suffer from gastrointestinal sequelae. This finding invites further exploration into the intricate interplay between vaccination, cytokine modulation, and gastrointestinal health.
Methods
Recruitment and sampling of vaccination cohorts
Twenty SARS-CoV-2 naïve healthcare workers from the University Hospital Halle (Saale), Germany, were recruited between December 2020 and January 2021 at the beginning of the German COVID-19 vaccination campaign to study vaccination efficiency after the initial mRNA vaccine rollout. Of these 20 individuals, 19 received the BioNTech-Pfizer Vaccine BNT162b2 (Tozinameran/Comirnaty), one the Moderna mRNA-1273 (Elasomeran/Spikevax) vaccine. As vaccination control, we used a cohort of additional 11 healthcare workers who received the seasonal influenza vaccine (VaxigripTetra 2020/2021) between October and November 2020 at the University Hospital Halle (Saale). Blood sampling of these vaccination cohorts was performed on the day of vaccine administration and four weeks later. The demographic characteristics of both vaccination cohorts are listed in Table 1. To assess the effect of post-infection vaccination on symptoms of post-COVID-19 condition, we used 540 individuals from the cohort study for digital health research in Germany (DigiHero) including 96 individuals without prior SARS-CoV-2 infection. Individuals were recruited between August 2021 and February 2022 via mailed invitation47. Participants completed an online questionnaire focusing on the detection and course of acute COVID-19, its sequelae, and vaccination status. Blood from the DigiHero participants was sampled once at a median of 8 months after the onset of the first COVID-19 symptoms. Demographic characteristics are listed in Table 2. The study was approved by the institutional review board (approval numbers approval number 2020-039 and 2020-076) and conducted in accordance with the ethical principles stated by the Declaration of Helsinki. Informed written consent was obtained from all participants or legal representatives. Plasma was isolated from whole blood via centrifugation of whole blood for 15 minutes at 2000 × g, followed by centrifugation at 12,000 × g for 10 minutes. All plasma samples were stored at - 80 °C before further use.
SARS-CoV-2 and influenza antibody profiling
Relative titers of antibodies targeting the S1 domain of the spike (S) protein and the nucleocapsid protein (NCP) of SARS-CoV-2 were determined using the Anti-SARS-CoV-2-ELISA IgA/IgG and Anti-SARS-CoV-2-NCP-ELISA kits from Euroimmun (Lübeck, Germany). ELISAs were coated with the respective recombinant antigen. To determine the relative titers of IgG class antibodies directed against influenza A and B, we used the Anti-Influenza-A-Virus-ELISA (IgG) and Anti-Influenza-B-Virus-ELISA (IgG) ELISA Kits from Euroimmun. Influenza A ELISA plates were coated with inactivated influenza A strains (Texas, H3N2; Singapore, H1N1; California, H1N1 (Porcine Influenza)) isolated from the allantoic fluid of infected chick embryos. In the case of influenza B, plates were coated with the inactivated virus of the B/Hong Kong/5/72 variant. Assays were performed according to the manufacturer’s instructions. Readouts were performed at 450 nm using a Tecan Spectrophotometer SpectraFluor Plus (Tecan Group Ltd., Männedorf, Switzerland).
Quantification of soluble factors in human plasma
Plasma cytokines were quantified using the LEGENDplex Human B Cell Panel (13-plex) and the Human Anti-Virus Response Panel (13-plex) (BioLegend) according to the manufacturer’s instructions. In addition, plasma levels of IL-5, IL-18, IL-23, IL-33, and CCL2/MCP-1 were quantified using the respective capture beads and corresponding detection antibodies from the LEGENDplex Human Inflammation Panel (Cat. No. 740809) and Human Th Panel (Cat. No. 741027) (BioLegend). Read out of the LEGENDplex assays was performed on a BD FACSCelesta. Concentrations were calculated using the LEGENDplex cloud-based Qognit Data Analysis Software (BioLegend). Heatmap of log-transformed plasma levels were generated with the R package pheatmap using the R version 4.3.1 and RStudio 2023.06.1.
Statistical analysis
Differences in plasma levels of antibodies or cytokines between the two groups were studied using the unpaired two-sided t-test. Comparisons between multiple groups were performed using ordinary one-way ANOVA followed by post-hoc testing (Tukey’s multiple comparisons test). The association between categorial symptom reporting and the number of post-infection vaccinations was tested with the chi-squared test for trend in proportions. All statistical analyses as well as the linear regression and Pearson correlation analyses for antibody plasma levels over time were performed using GraphPad PRISM 9.5.1 (GraphPad Software, La Jolla, CA, USA). Heatmaps were generated with the package pheatmap using R version 4.3.1 and RStudio 2023.06.1. Ranges of p values are indicated with asterisks: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The raw data supporting the conclusions of the study are available from the corresponding author.
References
Merad, M., Blish, C. A., Sallusto, F. & Iwasaki, A. The immunology and immunopathology of COVID-19. Science 375, 1122–1127 (2022).
Osuchowski, M. F. et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 9, 622–642 (2021).
Lamers, M. M. & Haagmans, B. L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 20, 270–284 (2022).
Chakraborty, S., Mallajosyula, V., Tato, C. M., Tan, G. S. & Wang, T. T. SARS-CoV-2 vaccines in advanced clinical trials: Where do we stand? Adv. Drug Deliv. Rev. 172, 314–338 (2021).
Voysey, M. et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397, 881–891 (2021).
Sadoff, J. et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 384, 2187–2201 (2021).
Polack, F. P. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Krammer, F. SARS-CoV-2 vaccines in development. Nature 586, 516–527 (2020).
Vogel, A. B. et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 592, 283–289 (2021).
Corbett, K. S. et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571 (2020).
Mathieu, E. et al. OurWorldInData.org (2020).
Sahin, U. et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 586, 594–599 (2020).
Widge, A. T. et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 384, 80–82 (2021).
Tarke, A. et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 185, 847–859.e811 (2022).
Garcia-Beltran, W. F. et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185, 457–466.e454 (2022).
Nunez, N. G. et al. High-dimensional analysis of 16 SARS-CoV-2 vaccine combinations reveals lymphocyte signatures correlating with immunogenicity. Nat. Immunol. 24, 941–954 (2023).
Andrews, N. et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 28, 831–837 (2022).
Pajon, R. et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N. Engl. J. Med. 386, 1088–1091 (2022).
Dagan, N. et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 384, 1412–1423 (2021).
Muik, A. et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 371, 1152–1153 (2021).
Paschold, L. et al. Rapid Hypermutation B Cell Trajectory Recruits Previously Primed B Cells Upon Third SARS-Cov-2 mRNA Vaccination. Front. Immunol. 13, 876306 (2022).
Reynolds, C. J. et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 372, 1418–1423 (2021).
Lyke, K. E. et al. Immunogenicity of NVX-CoV2373 heterologous boost against SARS-CoV-2 variants. NPJ Vaccines 8, 98 (2023).
Springer, D. N. et al. Bivalent COVID-19 mRNA booster vaccination (BA.1 or BA.4/BA.5) increases neutralization of matched Omicron variants. NPJ Vaccines 8, 110 (2023).
Sitaras, I. et al. Systematic review of primary and booster COVID-19 sera neutralizing ability against SARS-CoV-2 omicron variant. NPJ Vaccines 7, 147 (2022).
Brinkkemper, M. et al. A third SARS-CoV-2 spike vaccination improves neutralization of variants-of-concern. NPJ Vaccines 6, 146 (2021).
Hall, V. et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl. J. Med. 386, 1207–1220 (2022).
Barnes, E. et al. SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat. Med. 29, 1760–1774 (2023).
Ehmsen, S. et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 39, 1034–1036 (2021).
Fendler, A. et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat. Rev. Clin. Oncol. 19, 385–401 (2022).
Mellinghoff, S. C. et al. SARS-CoV-2 specific cellular response following COVID-19 vaccination in patients with chronic lymphocytic leukemia. Leukemia 36, 562–565 (2022).
Keppler-Hafkemeyer, A. et al. Potent high-avidity neutralizing antibodies and T cell responses after COVID-19 vaccination in individuals with B cell lymphoma and multiple myeloma. Nat. Cancer 4, 81–95 (2023).
Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 22, 277–282 (2022).
Nalbandian, A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615 (2021).
Mehandru, S. & Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 23, 194–202 (2022).
Altmann, D. M., Whettlock, E. M., Liu, S., Arachchillage, D. J. & Boyton, R. J. The immunology of long COVID. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-023-00904-7 (2023).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Nalbandian, A., Desai, A. D. & Wan, E. Y. Post-COVID-19 condition. Annu. Rev. Med. 74, 55–64 (2023).
Brannock, M. D. et al. Long COVID risk and pre-COVID vaccination in an EHR-based cohort study from the RECOVER program. Nat. Commun. 14, 2914 (2023).
Al-Aly, Z., Bowe, B. & **e, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28, 1461–1467 (2022).
Ayoubkhani, D. et al. Risk of long COVID in people infected with severe acute respiratory syndrome coronavirus 2 after 2 doses of a coronavirus disease 2019 vaccine: community-based, matched cohort study. Open Forum Infect. Dis. 9, ofac464 (2022).
Antonelli, M. et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. 22, 43–55 (2022).
Azzolini, E. et al. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA 328, 676–678 (2022).
Diexer, S. et al. Association between virus variants, vaccination, previous infections, and post COVID-19 Risk. Int. J. Infect. Dis. https://doi.org/10.1016/j.ijid.2023.08.019 (2023).
Byambasuren, O., Stehlik, P., Clark, J., Alcorn, K. & Glasziou, P. Effect of covid-19 vaccination on long covid: systematic review. BMJ Med. 2, e000385 (2023).
Schultheiss, C. et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 3, 100663 (2022).
Wisnivesky, J. P. et al. Association of vaccination with the persistence of post-COVID symptoms. J. Gen. Intern. Med. 37, 1748–1753 (2022).
Wynberg, E. et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study. Vaccine 40, 4424–4431 (2022).
Tsuchida, T. et al. Relationship between changes in symptoms and antibody titers after a single vaccination in patients with Long COVID. J. Med. Virol. 94, 3416–3420 (2022).
Klein, J. et al. Distinguishing features of Long COVID identified through immune profiling. medRxiv, 2022.2008.2009.22278592 https://doi.org/10.1101/2022.08.09.22278592 (2022).
Evans, R. A. et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir. Med. 9, 1275–1287 (2021).
Talla, A. et al. Persistent serum protein signatures define an inflammatory subcategory of long COVID. Nat. Commun. 14, 3417 (2023).
Reese, J. T. et al. Generalisable long COVID subtypes: findings from the NIH N3C and RECOVER programmes. EBioMedicine 87, 104413 (2023).
Schultheiss, C. et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J. Med. Virol. 95, e28364 (2023).
Kervevan, J. et al. Divergent adaptive immune responses define two types of long COVID. Front. Immunol. 14 https://doi.org/10.3389/fimmu.2023.1221961 (2023).
Zhang, H. et al. Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat. Med. 29, 226–235 (2023).
Etter, M. M. et al. Severe Neuro-COVID is associated with peripheral immune signatures, autoimmunity and neurodegeneration: a prospective cross-sectional study. Nat. Commun. 13, 6777 (2022).
Mateu, L. et al. Determinants of the onset and prognosis of the post-COVID-19 condition: a 2-year prospective observational cohort study. The Lancet Regional Health – Europe https://doi.org/10.1016/j.lanepe.2023.100724
Lazarus, J. V. et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat. Med. 29, 366–375 (2023).
Pierri, F. et al. Online misinformation is linked to early COVID-19 vaccination hesitancy and refusal. Sci. Rep. 12, 5966 (2022).
Schultheiss, C. et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease. Immunity 53, 442–455.e444 (2020).
Schultheiß, C. et al. Maturation trajectories and transcriptional landscape of plasmablasts and autoreactive B cells in COVID-19. iScience https://doi.org/10.1016/j.isci.2021.103325 (2021).
Woodruff, M. C. et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat. Commun. 14, 4201 (2023).
Phetsouphanh, C. et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 23, 210–216 (2022).
Kovarik, J. J. et al. A multi-omics based anti-inflammatory immune signature characterizes long COVID-19 syndrome. iScience 26, 105717 (2023).
Altmann, D. M. & Boyton, R. J. COVID-19 vaccination: The road ahead. Science 375, 1127–1132 (2022).
Sahin, U. et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 595, 572–577 (2021).
Jalkanen, P. et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat. Commun. 12, 3991 (2021).
Roltgen, K. et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 185, 1025–1040.e1014 (2022).
Muik, A. et al. Omicron BA.2 breakthrough infection enhances cross-neutralization of BA.2.12.1 and BA.4/BA.5. Sci. Immunol. 7, eade2283 (2022).
Regev-Yochay, G. et al. Correlates of protection against COVID-19 infection and intensity of symptomatic disease in vaccinated individuals exposed to SARS-CoV-2 in households in Israel (ICoFS): a prospective cohort study. Lancet Microbe 4, e309–e318 (2023).
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 375, 43–50 (2022).
Naranbhai, V. et al. Neutralization breadth of SARS-CoV-2 viral variants following primary series and booster SARS-CoV-2 vaccines in patients with cancer. Cancer Cell 40, 103–108.e102 (2022).
Kaneko, N. et al. Loss of Bcl-6-Expressing T follicular helper cells and germinal centers in COVID-19. Cell 183, 143–157.e113 (2020).
Turner, J. S. et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 596, 109–113 (2021).
Andrews, N. et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 386, 1532–1546 (2022).
Zollner, A. et al. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 163, 495–506.e498 (2022).
Sun, J. et al. Prolonged persistence of SARS-CoV-2 RNA in body fluids. Emerg Infect Dis 26, 1834–1838 (2020).
Swank, Z. et al. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciac722 (2022).
Noval Rivas, M., Porritt, R. A., Cheng, M. H., Bahar, I. & Arditi, M. Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis. Front. Immunol. 13, 941009 (2022).
Porritt, R. A. et al. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J. Clin. Invest. 131 https://doi.org/10.1172/JCI146614 (2021).
Vibholm, L. K. et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine 64, 103230 (2021).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185, 881–895.e820 (2022).
Peluso, M. J. et al. Multimodal Molecular Imaging Reveals Tissue-Based T Cell Activation and Viral RNA Persistence for Up to 2 Years Following COVID-19. medRxiv, 2023.2007.2027.23293177 https://doi.org/10.1101/2023.07.27.23293177 (2023).
Proal, A. D. et al. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat. Immunol. https://doi.org/10.1038/s41590-023-01601-2 (2023).
Gaebler, C. et al. Evolution of antibody immunity to SARS-CoV-2. Nature 591, 639–644 (2021).
Natarajan, A. et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 3, 371–387.e379 (2022).
Hu, F. et al. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell Mol. Immunol. 17, 1119–1125 (2020).
Garcia-Abellan, J. et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a Longitudinal Study. J. Clin. Immunol. 41, 1490–1501 (2021).
Lucke, J. et al. Intestinal IL-1beta Plays a Role in Protecting against SARS-CoV-2 Infection. J. Immunol. https://doi.org/10.4049/jimmunol.2200844 (2023).
Li, Y. et al. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target Ther. 6, 247 (2021).
Acknowledgements
The DigiHero consortium: Mascha Binder, Thomas Frese, Michael Gekle, Matthias Girndt, Jessica I. Höll, Rafael Mikolajczyk, Matthias Richter and Daniel Sedding. We thank Aline Patzschke, Christoph Wosiek, and Bianca Gebhardt for excellent technical assistance. We sincerely thank all probands and patients for participating in this study. We thank Alexander Navarrete-Santos and the flow cytometry core facility of the UKH for assistance with data acquisition. The schematic representation of the DigiHero sampling (Fig. 2a) was created using BioRender.com. This project was partially funded by the CRC 841 of the German Research Foundation (to MB) as well as by the Medical Faculty of the Martin-Luther-University Halle (Saale).
Author information
Authors and Affiliations
Contributions
M. Binder, R. Mikolajczyk, M. Gekle, C. Schultheiß, L. Paschold, C. Gottschick, B. Klee, S. Diexer, M. Girndt, T. Frese, D. Sedding and J. I. Höll designed the COVID-19 module of the DigiHero cohort study. D. Sedding and J. Dutzmann provided the HACO patient cohort. C. Fischer, C. Schultheiß and L. Paschold conducted experiments. C. Schultheiß, M. Binder, L. Paschold, E. Willscher, C. Fischer, L. Bosurgi, M. Addo and JszW analyzed and interpreted the data. C. Schultheiß and M. Binder drafted the manuscript. All authors critically revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fischer, C., Willscher, E., Paschold, L. et al. SARS-CoV-2 vaccination may mitigate dysregulation of IL-1/IL-18 and gastrointestinal symptoms of the post-COVID-19 condition. npj Vaccines 9, 23 (2024). https://doi.org/10.1038/s41541-024-00815-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-00815-1
- Springer Nature Limited