Abstract
To study the oxidation behavior of a commonly used pipeline materials of 37Mn5 steel in the process of fire flooding technology, a simulation device of organic fuel combustion was designed and used for comparative study between air and kerosene atmosphere. The products generated after kerosene combustion greatly affected the growth of iron oxides at 600 and 700 °C. In air, the oxide scale shows a defective morphology with bulge and spallation. While in the kerosene atmosphere, carbon particles after incomplete combustion can exist on the surface and inside the oxide scale, which would inhibit the reduction of oxides by carbon in substrate and the decarburization of 37Mn5 steel.
Similar content being viewed by others
Introduction
Crude oil, which accounts for more than 25–30% of the total petroleum, is one of the most important energy resources in the world1. Its production usually faces serious problems caused by low efficiency, especially in the case of heavy oil fractions2. Modern fire flooding technologies with low energy consumption and high efficiency currently represent the most suitable method for heavy oil extraction3,4. As the fire flooding proceeds at 400–700 °C, a gas mixture of CO, CO2, and H2O will be produced after oil combustion, which will then react with pipeline steels5,6. In a high-temperature environment, the service life of the steel materials depends not only on their mechanical properties, but also on the corrosion resistance. Therefore, studies of their corrosion behavior and mechanism in high-temperature environments have attracted worldwide attention7.
In general, organic fuel burns in air to form water vapor and carbon dioxide. In addition, some carbonaceous substances and other impurities will be present if the fuel is not fully combusted. Thus, a more complex high-temperature oxidation behavior would be expected for metallic materials oxidized in combustion gas than in air. In recent years, numerous studies have focused on the high-temperature oxidation behavior of steel materials in water vapor, carbon dioxide, or supercritical environments8,9,10,11. It is generally believed that the presence of water vapor accelerates the growth of oxide scales. For example, Nguyen et al.8 reported that water vapor accelerated the corrosion of Fe-9Cr alloys and hastened the onset of breakaway oxidation. Yuan et al.9 found that the oxide scale formed in water vapor was 20 μm thicker than that produced in oxygen, and its oxidation kinetics curve followed a linear growth law. Moreover, others proposed the microchannels in the oxide scale provide a diffusion pathway for H2 and/or H2O to ionize in the gap, accelerating the oxidation process10,11. Up to now, the accelerating mechanism of water vapor to high-temperature oxidation has not reached a unified theory.
Apart from the products of water vapor after fuel combustion process, carbides in various forms always play important roles on the oxidation behavior of steels. It is worth noting that CO2, as well as CO will react easily with the constituent elements of the oxide or substrate, especially when serving at high-temperature environment. For example, Huenert and Kranzmann11 proposed that steel would form carbide Fe3C upon oxidation in an atmosphere with high carbon activity. Other researchers reported that Fe-9Cr alloy would form a Cr-rich M23C6 phase when oxidized in a CO2 atmosphere at 800 °C12,13. Moreover, when two kinds of Nb modified Fe-25Cr-35Ni alloys were exposed to CO/CO2 gases at 982 and 1080 °C, both alloys underwent rapid internal carburization, indicating that their oxide scales failed to prevent carbon access to the underlying alloys under these reaction conditions14. Hence the mechanical performance and microstructural stability of alloys are expected to be greatly influenced by the decarbonization and reduction reactions of carbon-rich gases.
Many studies have focused on the metallic materials approaching equilibrium in a single atmosphere with H2O, CO2 or other reaction atmosphere. Their oxidation mechanism and behavior have been experimentally concluded on high-temperature environment. But under a multicomponent atmosphere, the research on the oxidation mechanism and corrosion behavior of alloys often face uncertain and complicated results15,16,17,18,19. For instance, the presence of sulfur-containing gas and oxygen could seriously reduce the protective properties of the oxide scales formed by Fe–Cr alloys at high temperature. However, when SO2 was added to CO–CO2–N2 gas at 900 °C, it will inhibit carbon penetration and carbide formation in high chromium alloys15,16,17. Moreover, when 304 stainless oxidized in CO/CO2, the presence of H2/H2O would change the reaction between the substrate and carbon: entirely internal carbide precipitation in CO/CO2, but principally graphite deposition in CO/H2/H2O18,19. Besides, only few research has been reported and focused on the environment of organic fuel combustion20,21. More details and impact mechanisms are not clear and need to be revealed. As a result, the study of the high-temperature oxidation behavior of Fe-based materials under special fuel environments remains to be continuous attention around worldwide.
According to American Petroleum Institute (API) 5L standard, 37Mn5 tube blank is mainly used to produce J55 grade steel pipe, which is one of the most widely used petroleum steel materials in fire flood applications22,23. Therefore, 37Mn5 steel was chosen as the substrate alloy in the present work. Figure 1 shows a schematic diagram of the reaction involved in the fire flooding technology. Fire flooding technology is to inject air into the oil layer and heat it downhole to a temperature that could ignite the crude oil. The ignition is implemented to realize underground combustion of the oil layer and decompose heavy oil usable light oil. In order to simulate the steel reaction state in the fuel oil environment as accurately as possible, a kerosene combustion device and was used to investigate the high-temperature oxidation behavior at a relatively low temperature of 600 °C, as well as at a practical temperature of 700 °C. Unlike the previous tube furnace where the synthesis atmosphere is introduced, the equipment is directly introduced with kerosene, and the kerosene burns at high temperature to produce corrosive gases. An oxidation experiment in air had been performed for comparison. The effect of the kerosene combustion atmosphere on the oxidation behavior of 37Mn5 steel (highlighted in blue in the reaction shown in Fig. 1) is discussed in detail. The effect of a high-salinity mineral fuel on the oxidation and hot corrosion behavior of pipeline steel (highlighted in yellow in Fig. 1) will be reported in detail in the next work, as well as other impurity gases, such as SO2 or H2S.
Results
High-temperature oxidation behavior of 37Mn5 steel at 600 °C
Figure 2a shows the oxidation kinetics of 37Mn5 steel after oxidation in air and kerosene atmosphere at 600 °C. All 37Mn5 steel samples showed a fast initial oxidation stage during the first 4 min. After that, the samples showed different trends. The mass gain of the steel samples in kerosene atmosphere maintained a rapid growth rate for the whole test. However, the specimens oxidized in air showed a relatively stable mass change for the next 26 min; the mass gain was 0.047 mg cm−2. In addition, the 37Mn5 steel samples in kerosene exhibited a higher mass gain of 0.207 mg cm−2, which was almost four times that in air.
In order to identify the phase constituents of the oxide scales formed in the two atmospheres, the XRD patterns measured after different time intervals are shown in Fig. 2b, c. In common to all patterns, the strongest peaks corresponded to the α-Fe matrix phase, from which it can be speculated that the formed oxide scale was relatively thin. The main oxide products of 37Mn5 steel in air and kerosene were Fe2O3 and Fe3O4. For the specimens oxidized in air, no obvious oxide peaks were detected during the first 6 min. As the oxidation experiments proceeded, the α-Fe peaks became weaker. In addition, two kinds of oxides were already formed in the first 6 min when the oxidation was carried out in kerosene atmosphere. This is also consistent with the higher mass gain of the 37Mn5 steels observed in the kinetic tests. As the oxidation time increased, the peaks of Fe2O3 and Fe3O4 became increasingly stronger. Interestingly, another Fe oxide, FeO, was detected after oxidation for 16 min in kerosene atmosphere.
The surface morphologies and cross-sectional microstructures of oxide scales formed in air and kerosene atmospheres are shown in Fig. 3, respectively. All steels clearly show high oxidation resistance at 600 °C, and no scale spallation occurred. In particularly, as shown in Fig. 3a, e, some grooves could be distinctly observed on the thin scales of the specimens after oxidation for 16 min, due to the polishing and grinding processes. The amplified view of the surface morphologies shows that shallower scratches were formed upon oxidation in kerosene than in air. However, hardly any scratches were observed on the surface of the steels oxidized in kerosene atmosphere for 30 min. Combined with the cross-sectional microstructure results, this can be mainly attributed to the different thicknesses of the oxide scales. As shown in Fig. 3e–h, the oxide scales formed in kerosene atmosphere were much thicker compared with those of the samples oxidized for the same time interval in air. It is worth noting that many pores were observed on the surface of the steel samples after oxidation in kerosene for 30 min.
a Surface microstructure for 16 min in air. b Surface microstructure for 30 min in air. c Surface microstructure for 16 min in kerosene. d Surface microstructure for 30 min in kerosene. e Cross-sectional microstructure for 16 min in air. f Cross-sectional microstructure for 30 min in air. g Cross-sectional microstructure for 16 min in kerosene. h Cross-sectional microstructure for 30 min in kerosene.
High-temperature oxidation behavior of 37Mn5 steel at 700 °C
When 37Mn5 steel was oxidized at a higher temperature of 700 °C, significant differences were found with the results obtained at 600 °C. Figure 4a shows the oxidation kinetics of 37Mn5 steel after oxidation in air and kerosene atmosphere at 700 °C. In this case, after only 4 min of oxidation the steels reached the total mass gain achieved in 30 min at 600 °C. Following the same trend observed at 600 °C, 37Mn5 steel showed a higher mass gain in kerosene atmosphere. Moreover, the mass gain was twice as high as that in air during the whole oxidation test: 0.259 and 0.586 mg cm−2 in air and kerosene atmosphere, respectively.
Figure 4b, c shows the XRD patterns of the 37Mn5 steels after oxidation at 700 °C. Three different types of Fe oxides were formed on the steels. The peaks of Fe2O3 and Fe3O4 were higher than those of the substrate α-Fe phase after 16 min of oxidation at 700 °C; FeO was found in both groups.
The surface morphologies of the oxide scales after oxidation in air and kerosene atmosphere for 16 and 30 min are shown in Fig. 5a–d. Few scratches and many microcracks, formed between neighboring slices, could be observed on the surface of the 37Mn5 steels oxidized in air for 16 min, as shown in Fig. 5a. As the oxidation time was extended to 30 min, the surface became rough and wrinkled. To investigate the detailed microstructure of the steels after oxidation, the cross-sectional images of the 37Mn5 steel after oxidation in air and kerosene atmosphere are shown in Fig. 5e–h. The results show that the oxide scales were much thicker than those observed at 600 °C. As shown in Fig. 5e, f, spallation areas and blisters were found on the surface of the oxide scales, which corresponded to their surface morphologies. The EDS results show that the scales were mainly composed of Fe2O3. Intact scales were observed for the specimens after oxidation in kerosene for 16 and 30 min, as shown in Fig. 5c, d. Many pores were formed when the samples were oxidized for 16 min. Moreover, a porous scale was observed in the cross-sectional morphologies shown in Fig. 5g, h. The oxide was mainly composed of Fe2O3, with small amounts of Fe3O4 and FeO.
a Surface microstructure for 16 min in air. b Surface microstructure for 30 min in air. c Surface microstructure for 16 min in kerosene. d Surface microstructure for 30 min in kerosene. e Cross-sectional microstructure for 16 min in air. f Cross-sectional microstructure for 30 min in air. g Cross-sectional microstructure for 16 min in kerosene. h Cross-sectional microstructure for 30 min in kerosene.
Figure 6 shows the depth distribution of Fe and O elements in 37Mn5 steels after oxidation in the two atmospheres for 30 min at 700 °C, as measured by GDOES. According to Fig. 6a, the Fe content of the oxide scales in air gradually increased from the outer to the inner region, whereas the O content showed the opposite trend. The Fe and O contents remained unchanged in the range of 1.5 to 10 μm. The thickness of the oxide scale in Fig. 5f was 2.1 μm, which is slightly different from this range, owing to a gap at the interface between the oxide scale and the substrate. Based on Fig. 6b, in the kerosene atmosphere the Fe and O contents changed rapidly in the range of 0–0.5 μm, with relatively huge fluctuations. In the range of 3–4.6 μm, the changes in the contents of Fe and O elements were very similar to those in air, and no clear boundary between the inner and outer oxide films could be observed in the element distribution. Although the phases of the oxide scales in the two atmosphere were different, the oxide scale near the substrate had the same composition. This was due to the presence of Fe3O4 precipitation in the inner oxide scales in the kerosene atmosphere, shown as Fig. 5h.
Discussion
During the kerosene combustion experiment, water vapor and carbon dioxide are generated after combustion. Owing to the filling of organic fuel, the combustion process always performs more fiercely than in air and is accompanied by incomplete combustion. Thus, carbon monoxide and solid carbon particles are the main oxidation products. As mentioned in the Introduction section, water vapor and carbon dioxide have a significant influence on the oxidation behavior of metals, which affects the diffusion of oxygen and the participation of other gases. On the other hand, carbon monoxide and carbon particles produced by incomplete combustion of kerosene could reduce oxides to metals. Therefore, 37Mn5 steel exhibits different oxidation behaviors under different conditions.
Oxidation behavior of 37Mn5 in air
For typical pipeline steels, there are different types of oxidation reactions in air. Chen and Yuen24 reported that different oxidation behaviors were observed below 550–600 °C; similar results were obtained in this work. The main oxidation product was ferric oxide. At oxidation temperatures above 600 °C, the products changed to another form of mixed oxide layer, containing Fe3O4, Fe2O3, and inner FeO (Figs. 2, 4). At different temperatures, the growth rate of the oxide scales at 700 °C is approximately twice that at 600 °C; more importantly, the oxidation products of the two systems changed significantly. The different activity and outward diffusion ability of Fe at different temperatures determined the difference in the formed oxidation products, as a result of the change in the outward diffusion of Fe and inward diffusion of oxygen. In addition, we found that the surface of the oxide scale bulged out and peeled off in air.
The spallation of oxidation products is often related to stress concentration25,26. When the volume of the consumed metal is larger than that of the oxide, growth stress will be generated in the oxide scale or in the steel. The Pilling–Bedworth ratio (PBR) represents the ratio of the oxide growth thickness ξ to the consumed metal substrate thickness y. When PBR > 1, the oxide film will be subjected to compressive stress, whereas when PBR < 1 the oxide film will experience tensile stress. In this case, the PBR values of both ferric and magnetite are greater than 1. Then the direction and magnitude of the stress are determined by the thickness of the oxide scale and consumed substrate, according to the following linear relation:
Фy is the thickness of the oxide scale. It has been reported that a higher stress is generated when the oxidation products are relatively thick27. However, the thicker oxide scale formed in kerosene atmosphere was found to be well bonded, without bulging and swelling. Another important factor is the difference between the coefficients of linear expansion (α) of the matrix (αm) and oxide scale (αox). Generally, αm is larger than αox. Therefore, when the oxidation temperature increases, the oxide film experiences tensile stress, whereas during the cooling process the oxide film is subjected to compressive stress28. The stress is proportional to the difference in the thermal expansion coefficients of the matrix and oxide scale, as described by the following equation:
Where αox and αm denote the thermal expansion coefficient of oxide and metal, respectively; ΔT is the temperature change; Eox and Em denote the Young’ s modulus of oxide and metal, respectively; ξ and h are the thickness of oxide and metal, respectively; v is the Poisson ratio. In general, the thickness of the oxide scale ξ is much lower than that of substrate h; hence, the above formula can be simplified to the following equation:
The above equations suggest that the thermal stress is not only positively proportional to the difference between the thermal expansion coefficients, but also increases with increasing temperature difference. For the same temperature difference value and oxides products (XRD analysis in Fig. 4) in two atmospheres at 700 °C, neither bulged nor spallation is observed on the specimens after kerosene combustion for 30 min. It is evidently show that the occurrence of spalling here in Fig. 5e, f is not significantly influenced by the linear expansion coefficient.
Table 1 shows that the carbon content in 37Mn5 steels is 0.34–0.39 wt.%. These carbon species mainly exist as Fe3C, which is a metastable phase and will decompose into iron and carbon at high temperature. Previous studies have shown that carbon at the oxide scale–substrate interface reacts with the oxides and causes carbon steels to decarburize at high temperature29. The carbon species formed by decomposition of Fe3C would diffuse to the oxide scales-substrate interface through the lattice and grain boundaries, before reacting with FeO:
Carbon monoxide escapes into the external atmosphere through microchannels or voids in the oxide scales. At the same time, Fe2+ also diffuses to the external surface and combines with oxygen ions to generate new oxides. Therefore, near the oxide scale–substrate interface, the Fe content of the substrate is decreased and that of carbon is slightly higher, which is consisted with the GDOES results and show in Fig. 6a. Carbon reduction occurs at the interface to form pores, and then carbon monoxide/dioxide gases will be released in the air. This also suggests that the surface of the sample would be enriched with elemental carbon after 30 min in air. During the oxidation process, growth and thermal stresses are generated on the oxide scales. Stress concentration would occur when the samples are cooled and the number of micropores is small, so that the stress would be difficult to release. When the stress concentration reaches a certain level, the oxide scales preferentially deform at micropores to form larger pores. As shown in Fig. 5, flawed microstructures with bulged and spallation areas are found on the 37Mn5 after oxidation in air at 700 °C.
Oxidation behavior of 37Mn5 in kerosene atmosphere
As mentioned above, 37Mn5 steel exhibits a severe and complex oxidation behavior in kerosene. According to the oxidation kinetics (Figs. 2, 4), at the same temperature, the rate of oxidation of 37Mn5 steel in kerosene atmosphere was higher than that in air. For example, at 600 °C, the weight gain of 37Mn5 steel in kerosene atmosphere was about four times that in air. Studies have shown that the presence of water vapor can accelerate the growth of iron oxides30,31. Rahmel and Tobolski30 proposed that the presence of water vapor would increase the oxidation rate by 1.6 times at 950 °C. At the same time, it has been proposed that water vapor could be transported into the oxide scales through microcracks. Douglass et al.31 suggested that the oxide scales contained pores and cracks that could not be observed by SEM. Generally, as shown in Fig. 5, the water vapor generated after kerosene is fully burned will react in small holes and cracks on the oxide scale with reactions (5) and (6):
where \(O_O^X\), \(V_{Fe}^{\prime\prime}\), and \(h^ \cdot\) represent oxygen ions, ferrous ion vacancies, and electron holes at normal oxygen sites, respectively. H2 is converted to H2O on the near-substrate side in the pores, while H2O is converted to H2 on the other (oxide scale) side. Therefore, reactions (5) and (6) describe the accelerated oxidation process of water vapor. Owing to the water vapor, the oxide scales were thicker than in air. In addition, Tuck et al.32 proposed that the oxidation of iron at 950 °C would make the peeling of oxide scales more difficult. According to the present results, the oxide scales formed by the 37Mn5 steels in the kerosene atmosphere were thicker but not easy to peel off; in other words, the presence of water vapor improved the adhesion of the grown oxide scales, which was attributed to the good plasticity of oxides caused by the pores.
Although FeO was found in the oxide scales after oxidation for 30 min at 600 °C in kerosene atmosphere, the kinetic rate did not change significantly. This is due to the indirect redox reaction inside the oxide scales. However, at a higher temperature (700 °C), FeO could be found in both air and kerosene atmospheres. When iron was oxidized at high temperature, Fe2O3 and Fe3O4 were formed first, followed by the reaction of Fe2+ with Fe3O4:
Therefore, Fe2O3 and Fe3O4 would be formed first, followed by FeO.
Studies have shown that the high-temperature oxidation of pure iron causes metal cations to diffuse outward, so that the oxide scales grow outward33,34. As mentioned above, incomplete combustion of kerosene generates solid carbon particles, and carbon adheres to the surface of the oxide scales. The carbon particles on the oxide scale surface would be wrapped into the scales, owing to their outward growth. Figure 6b shows that the atomic percentage of elemental carbon near the surface of the oxide scales reached ~21 at. %, while the carbon content in the substrate was only 1.5 at. %, which also indicates enrichment in carbon particles on the surface of the oxide scales. Carbon will undergo reduction reactions with the iron oxides, such as (8) and (9):
in which carbon reduces Fe2O3, Fe3O4, and FeO to Fe3O4, FeO, and Fe, respectively. Fe3+ would be reduced to Fe2+, with carbon as the reducing agent. As Fe3+ would be preferentially reduced by the wrapped carbon; therefore, the pores formed by gas release could make the oxide scales loose, as shown in Fig. 5g, h. As the oxidation proceed, carbon was adsorbed on the surface of the sample, where oxide scale was looser and more porous than the internal oxide scales. Then the oxide scales kept grow outward and pores were formed on the newly formed scales, which cycling became loose and porous due to the adsorption of carbon particles. Besides, it is due to the presence of reduced carbon formed by incomplete combustion, which inhibits the decarburization reaction (4) of the matrix, and thus does not produce large defects such as bulging and spalling. In fact, the amount of absorbed carbon is not sufficient for complete reduction as present in reaction (8) and (9). Then the residual Fe2O3 and Fe3O4 will be reduced to Fe2+, which will diffuse outward and oxidize subsequently. The Fe2O3 and Fe3O4 species closer to the substrate will be preferentially reduced to FeO, so that the precipitated Fe2O3 and Fe3O4 surrounded by FeO will eventually become FeO scales but without Fe2O3 and Fe3O4. This can also be explained by the distribution of iron and oxygen elements along the depth of the oxide scales in the region of 0.8–3 μm, shown in Fig. 6b.
Schematic diagram
Figures 7, 8 show the schematic diagram of the microstructure evolution of 37Mn5 steels after oxidation in air and kerosene combustion atmosphere at 700 °C, respectively. During oxidizing in different environment, especially in kerosene combustion atmosphere, carbon or carbides always induce a significant influence on the growth structure of oxide scale. In most carbon steel materials, there normally contain many carbon-rich phases (Fig. 7a) in the matrix. Moreover, the matrix usually suffers a decarbonization process at high temperature, resulting in the reduction and decomposition of the oxide scale. Finally, gas will be generated and released from the surface oxide layer, and then defects morphologies are formed (Fig. 7b).
Different from that in air, some incomplete burned carbon particles are always occupied and contained in the oxide scale after combustion in kerosene (Fig. 8a). These carbides and carbon oxides reduce the activity of C in the surface of steel / oxide, thus inhibiting the decarburization reaction. However, the reduction reaction still takes place in the inner part of the oxide scale at first, and with the extension of oxidation time, it will gradually occur and affect on the outermost surface (Fig. 8b, c). Some internal micropores will gradually close by the bidirectional diffusion reaction of iron and oxygen. These pores also provide sites for the accelerated oxidation of water mentioned in above 4.2 part (Fig. 8d).
Above all, the combustion of heavy oil tends to accelerate the oxidation of mining pipelines in the process of modern fire flooding. It is corresponding to test results in kerosene atmosphere for 30 min, the weight gain of it has doubled compared to that in air. What’s more, the accelerated failure process of pipelines is one-sided to solve the problem on considering only in the combustion atmosphere. The presence of salt, such as sulfosalt, will greatly accelerate the corrosion of the pipeline, even more serious than the combustion atmosphere, as shown in Fig. 1 (highlighted in yellow). The effect of salt on accelerating the corrosion process of pipeline steels will be considered in the next topic. The main conclusions are as follows:
-
(1)
A kerosene combustion device is invented to investigate the high-temperature oxidation behavior at a relatively low temperature of 600 °C, as well as at a practical temperature of 700 °C. Oxidation kinetics results show the weight gain of 37Mn5 steel is higher than that in air. All 37Mn5 steels show a fast initial oxidation stage after 4 min. The weight gain of 37Mn5 steel in kerosene atmosphere is about four times, two times larger than that in air at 600 and 700 °C, respectively.
-
(2)
Carbon exhibits different effects in the two oxidation environments. In air, the structure of oxide scale is affected by decarburization reaction in the matrix, forming defective bulge expansion and spalling failure; in kerosene combustion environment, the decarburization reaction in the matrix is inhibited by the external C. But a loose oxide scale and pores are observed on the surface by reduction reaction between C/COx(x = 1, 2) and iron oxides.
-
(3)
The water vapor generate after kerosene combustion can accelerate the growth of iron oxides. The presence of water vapor improves the adhesion of the oxide scales.
Methods
Materials
The 37Mn5 billets were produced by the Institute of Metal Research (IMR), Chinese Academy of Science under the steady casting condition. The composition of the 37Mn5 pipeline steel is shown in Table 1. The cast 37Mn5 steel was machined into specimens of 20 × 20 × 2 mm dimensions. Samples were ground with 150, 400, 800, and 1000 SiC sandpaper, and then degreased by an ultrasonic cleaner in acetone and ethanol for 15 min.
Kerosene combustion oxidation
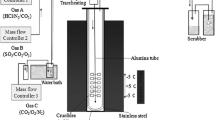
During the combustion experiment, 1# aviation kerosene (heavy oil produced by Hongrui Petrochemicals Co., Ltd, Shenyang, China) was used to simulate the combustion of heavy oil35. A new kerosene combustion device was built to simulate the oxidation experiments in the relevant atmosphere. Figure 9 shows a schematic diagram of the kerosene combustion device. The device was mainly composed of three parts: fuel supply, combustion, and exhaust gas treatment systems. First, the fuel supply system delivered kerosene to a high-temperature furnace through a power pump. The flow rate of kerosene in the combustion system was controlled at 5 ml min−1. In addition, the combustion system was mainly composed of a tube furnace and a reaction chamber. Comparative high-temperature oxidation experiments were conducted in the tube furnace, both in air and kerosene at 600 and 700 °C for 30 min. Before the experiment, the temperature of the constant-temperature zone was calibrated with an S-type platinum-rhodium thermocouple. During firing and the subsequent oxidation processes, all specimens were fixed on a Ni-Cr frame and transferred into a reaction chamber pre-heated to 600 or 700 °C. As soon as the specimens entered the reaction chamber, kerosene was trickled from the supply system to the combustion system, and timing was started. After appropriate time intervals of 2 min, the samples were removed from the furnace, cooled in air, and then weighed using an electronic balance with a sensitivity of 0.01 mg. The samples were cleaned using deionized water before weighing. To ensure reliability, each group included three parallel specimens. Finally, the exhaust gas treatment system was mainly composed of a power pump and a container loaded with the waste gas treatment liquid. The gas mixture was discharged into the system through the end of the tube furnace.
Characterization
The phase composition was characterized by X-ray diffraction (XRD, X’Pert PRO, PANalytical Co., Almelo, Holland, Cu Ka radiation at 40 kV). The obtained XRD patterns were recorded in the 2θ range of 10–90°, in step-scanning mode with a step size of 0.02°. The surface and cross-sectional morphologies and microstructures of the oxidized samples were examined by scanning electron microscopy (SEM, Inspect F50, FEI Co., Hillsboro, Oregon) coupled with energy-dispersive spectrometry (EDS, X-Max, Oxford instruments Co., Oxford, UK). For the cross-sectional SEM characterization, in order to maintain the original appearance of the oxide scales, the samples were not coated in some way before the cross-sectioning. The depth distribution of elements was measured by glow discharge optical emission spectrometry (GDOES, GDA750HP, Spectruma Analytik GmbH, Germany).
Data availability
All data included in this study are available upon request by contact with the corresponding author.
References
Nabipour, M. et al. Laboratory investigation of thermally-assisted gas-oil gravity drainage for secondary and tertiary oil recovery in fractured models. J. Pet. Sci. Eng. 55, 74–82 (2007).
Ancheyta, J., Sanchez, S. & Rodriguez, M. A. Kinetic modeling of hydrocracking of heavy oil fractions: a review. Catal. Today 109, 76–92 (2005).
Yuan, S. et al. A new description method of the position of combustion front in dry linear fire flooding process. Int. Commun. Heat. Mass Transf. 113, 104530 (2020).
Li, Q. et al. Mechanisms and influencing factors of the oil bank in fire flooding. Pet. Explor. Dev. 45, 491–498 (2018).
Kumar, M. & Garon, A. M. An experimental investigation of the fireflooding combustion zone. SPE Reserv. Eng. 6, 55–61 (1991).
Shahani, G. H. & Hansel, J. G. Oxygen fireflood: Combustion tube tests with light, medium, and heavy crude oils. SPE Reserv. Eng. 2, 583–590 (1987).
Li, L., Jiang, Z. & Riquier, Y. High-temperature oxidation of duplex stainless steels in air and mixed gas of air and CH4. Corros. Sci. 47, 57–68 (2005).
Nguyen, T. D., Zhang, J. & Young, D. J. Water vapour effects on corrosion of Fe-Cr and Fe-Cr-Ni alloys containing cerium and manganese in CO2 gas at 818oC. Corros. Sci. 89, 220–235 (2014).
Yuan, J., Wang, W., Zhu, S. & Wang, F. Comparison between the oxidation of iron in oxygen and in steam at 650-750oC. Corros. Sci. 75, 309–317 (2013).
Saunders, S. R. J., Monteiro, M. & Rizzo, F. The oxidation behavior of metals and alloys at high temperatures in atmospheres containing water vapour: A review. Prog. Mater. Sci. 53, 775–837 (2008).
Huenert, D. & Kranzmann, A. Impact of oxyfuel atmospheres H2O/CO2/O2 and H2O/CO2 on the oxidation of ferritic–martensitic and austenitic steels. Corros. Sci. 53, 2306–2317 (2011).
Gheno, T., Monceau, D., Zhang, J. & Young, D. J. Carburisation of ferritic Fe–Cr alloys by low carbon activity gases. Corros. Sci. 53, 2767–2777 (2011).
Shen, J., Liu, S., Guo, X. H. & Niu, Y. Simultaneous oxidation and carburization of a Fe-9Cr alloy under different oxygen pressures at 800°C. Corros. Sci. 129, 1–15 (2017).
Xu, N., Monceau, D., Young, D. & Furtado, J. High temperature corrosion of cast heat resisting steels in CO+CO2 gas mixtures. Corros. Sci. 50, 2398–2406 (2008).
Mrowec, S. The problem of sulfur in high-temperature corrosion. Oxid. Met. 44, 177–209 (1995).
Liu, S., Shen, J., Wu, X. F., Li, H. & Niu, Y. The effect of sulphur on the carburization of three Fe-19Ni-13Cr alloys with various Al additions at 900oC in oxygen-contaminated CH4-H2-H2S atmospheres. Corros. Sci. 112, 94–109 (2016).
Moszynski, D., Grabke, H. J. & Schneider, A. Effect of sulphur on the formation of graphite at the surface of carbusized iron. Surf. Interface Anal. 34, 380–383 (2020).
Zhang, J., Boddington, K. & Young, D. Oxidation, carburization and metal dusting of 304 stainless steel in CO/CO2 and CO/H2/H2O gas mixtures. Corros. Sci. 50, 3107–3115 (2008).
Li, H., Zhang, J. & Young, D. Oxidation of Fe-Si, Fe-Al and Fe-Si-Al alloys in CO2- H2O gas at 800oC. Corros. Sci. 54, 127–138 (2012).
**e, D., Shan, G. & Lv, S. Oxidation behavior of carbon steel in simulated kerosene combustion atmosphere: A valuable tool for fire investigations. Fire Mater. 42, 156–163 (2017).
**, P. & Nesic, S. Mechanism of magnetite formation in high temperature naphthenic acid corrosion by crude oil fractions. Corros. Sci. 115, 93–105 (2017).
Wang, Y. et al. Mechanism and control of sulfide inclusion accumulation in CET Zone of 37Mn5 round billet. Metall. Mater. Trans. B 48, 1004–1013 (2017).
Singh, A. et al. Gingko biloba fruit extract as an eco-friendly corrosion inhibitor for J55 steel in CO2 saturated 3.5 % NaCl solution. J. Ind. Eng. Chem. 24, 219–228 (2015).
Chen, R. Y. & Yuen, W. Y. D. Review of the high temperature oxidation of iron and carbon steel in air or oxygen. Oxid. Met. 59, 433–468 (2003).
Yang, L., Zheng, L. & Guo, H. The residual stress of oxide scales grown on Ni-Al alloys doped with minor Dy and Y. Corros. Sci. 112, 542–552 (2016).
Selcuk, A. & Atkinson, A. The evolution of residual stress in the thermally grown oxide on Pt diffusion bond coats in TBCs. Acta Mater. 51, 535–549 (2003).
Christensen, R. J., Lipkin, D. M. & Clarke, D. R. The stress and spalling behavior of the oxide scale formed on polycrystalline Ni3Al. Acta Mater. 44, 3813–3821 (1996).
Yang, S. et al. Understanding of failure mechanisms of the oxide scales formed on nanocrystalline coating with different Al content during cyclic oxidation. Acta Mater. 205, 116576 (2021).
Li, D. et al. Investigation of decarburization in spring steel production process-part I: experiments. Steel Res. Int. 80, 304–310 (2010).
Rahmel, A. & Tobolski, J. Einfluss von was serdampf und kohlendioxyd auf die oxydation von nickel in sauerstoff bei hohen temperature. Corros. Sci. 5, 815–820 (1965).
Douglass, D., Kofstad, P., Rahmel, A. & Wood, G. C. International workshop on high-temperature corrosion. Oxid. Met. 45, 529–620 (1996).
Tuck, C., Odgers, M. & Sachs, K. The oxidation of iron at 950oC in oxygen/water vapour mixtures. Corros. Sci. 9, 271–280 (1969).
Baud, J., Ferrier, A., Manenc, J. & Bebard, J. The oxidation and decarburizing of Fe–C alloys in air and the influence of relative humidity. Oxid. Met. 9, 69–97 (1975).
Turkdogan, E. T., Mckewan, W. M. & Zwell, L. Rate of oxidation of Iron to wustite in water-hydrogen gas mixtures. J. Phys. Chem. 69, 327–334 (1965).
**e, D., Shan, G., Deng, S. & Liu, L. Investigations on oxidation and microstructure evolution of pure Cu in simulated air-kerosene combustion atmosphere. Fire Mater. 41, 614–624 (2017).
Acknowledgements
This project is financially supported by the National Natural Science Foundation of China under Grant (51671053 and 51801021), the National Key R&D Program of China under Grant (No. 2017YFB0306100), the Fundamental Research Funds for the Central Universities (No. N2102015), and by the Ministry of Industry and Information Technology Project (No. MJ-2017-J-99).
Author information
Authors and Affiliations
Contributions
B.M.: conceptualization, methodology, investigation, and writing. J.W.: Writing—review and editing, formal analysis, investigation. L.Y.: Writing—review and editing, investigation. M.C.: additive manufacturing, resources, funding acquisition, and resources. S.Z. and F.W.: supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meng, B., Wang, J., Yang, L. et al. Influence of fuel combustion on the corrosion behavior of pipeline steels in fire flooding technology. npj Mater Degrad 6, 21 (2022). https://doi.org/10.1038/s41529-022-00230-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-022-00230-8
- Springer Nature Limited