Abstract
The use of solid-oxide materials in electrocatalysis applications, especially in hydrogen-evolution reactions, is promising. However, further improvements are warranted to overcome the fundamental bottlenecks to enhancing the performance of solid-oxide electrolysis cells (SOECs), which is directly linked to the more-refined fundamental understanding of complex physical and chemical phenomena and mass exchanges that take place at the surfaces and in the bulk of electrocatalysis materials. Here, we developed an eReaxFF force field for barium zirconate doped with 20 mol% of yttrium, BaZr0.8Y0.2O3-δ (BZY20) to enable a systematic, large-length-scale, and longer-timescale atomistic simulation of solid-oxide electrocatalysis for hydrogen generation. All parameters for the eReaxFF were optimized to reproduce quantum-mechanical (QM) calculations on relevant condensed phase and cluster systems describing oxygen vacancies, vacancy migrations, electron localization, water adsorption, water splitting, and hydrogen generation on the surfaces of the BZY20 solid oxide. Using the developed force field, we performed both zero-voltage (excess electrons absent) and non-zero-voltage (excess electrons present) molecular dynamics simulations to observe water adsorption, water splitting, proton migration, oxygen-vacancy migrations, and eventual hydrogen-production reactions. Based on investigations offered in the present study, we conclude that the eReaxFF force field-based approach can enable computationally efficient simulations for electron conductivity, electron leakage, and other non-zero-voltage effects on the solid oxide materials using the explicit-electron concept. Moreover, we demonstrate how the eReaxFF force field-based atomistic-simulation approach can enhance our understanding of processes in SOEC applications and potentially other renewable-energy applications.
Similar content being viewed by others
Introduction
Electrocatalysis is a catalytic process in which the rate of an electrochemical reaction occurring at the electrode-electrolyte interface can be controlled by varying the electrical potential. The electrocatalyst is an indispensable component of electrocatalysis processes, a backbone of various electrochemical devices, that facilitates complex oxidation and reduction reactions through a direct transfer of electrons and ions, and manifests in secondary-reaction products. Ideally, the electrocatalysts must function efficiently and should possess an ability to lower the overpotential (or the electrochemical-reaction barrier) of such oxidation and reduction reactions1, adsorb key intermediate species, and facilitate alternate energy pathways for the evolution of reaction products2. Such operational requirements are prevalent in many renewable-energy applications, including fuel cells, solid-oxide electrolysis cells (SOECs), and electrochemical energy-storage devices such as metal–air batteries3.
Among various electrocatalysis-reaction pathways, hydrogen-production pathways based on the water-splitting reaction, as opposed to reforming hydrocarbons, are considered attractive and promising for a sustainable future4,5. A typical water-splitting process consists of employing electricity generated from renewable energy sources to split pure water into oxygen and hydrogen6. The water-splitting reaction, which consists of the hydrogen-evolution reaction (HER; 2H+ + 2e−→H2), is highly relevant for the production of hydrogen7,8, where an electrocatalyst material could play a pivotal role. Electrocatalysts can manifest in various material compositions, including metals, metal oxides, and carbonaceous materials9. These materials have been used in various hydrogen generation technologies, such as alkaline10,11, proton-exchange membrane6,12, and SOECs13,14. At present, transition metal oxides stand out as highly favorable electrocatalysts for hydrogen-evolution reaction (HER). Barium zirconate doped with 20 mol% of yttrium, BaZr0.8Y0.2O3-δ (BZY20) emerges as a nearly pure proton conductor in environments containing water vapor and/or hydrogen at temperatures below 650 °C. BZY20 exhibits high proton conductivity with minimal energy barrier15, and it maintains exceptional chemical stability16. Nevertheless, its electrical efficiency is compromised by electronic leakage17. Among different technologies, SOECs are the least-developed technologies because most past efforts have been focused on mitigating various challenges associated with the SOEC operation and lab demonstration of SOEC technology18,19,20. Significant fundamental advances were needed to resolve the fundamental bottlenecks in SOECs to accelerate their widespread adoptability and affordability, as described in the recent US Department of Energy (DOE) 1:1:1 initiative (https://www.energy.gov/eere/fuelcells/hydrogen-shot).
The reaction rate of an electrocatalyst system can be improved by increasing the number of active sites on a given electrode—e.g., through increased loading or improved catalyst structuring to expose more active sites to facilitate binding different reaction intermediates and transition states in different ways. This can be done by alloying, do**, or introducing defects. Recently, we reviewed various operating and materials factors affecting the faradaic efficiency of SOECs and computational advances needed to model various SOEC intrinsic processes that would complement ongoing experimental efforts and generate groundbreaking new insights to improve SOEC faradaic efficiency21.
Due to the limitation of such desperately needed fundamental research advances, here we describe the development and application of an eReaxFF force field-based reactive molecular dynamics simulation as an approach to systematically delineate various chemical and electrochemical reactions—oxygen-vacancy migrations, water adsorption, and water splitting at different surfaces—which are central to hydrogen formation and linked to the performance and faradaic efficiency of SOECs. We used a typical BZY20 solid-oxide material, as an electrocatalyst in our research pursuit to demonstrate the eReaxFF interatomic-potential development approach and the utility prospects of the newly developed eReaxFF force field in resolving fundamental bottlenecks in SOEC material design and optimization processes. eReaxFF is 6 or more magnitudes faster than time-dependent density functional theory simulations—which are typically required to simulate reactions with an explicit electrochemical component—enabling us to simulate realistic, >1000 atom systems at a relatively low computational cost. Furthermore, we also discuss the extension of the eReaxFF force field-based approach to other renewable-energy applications, including lithium-metal batteries, solid-state batteries, and solid-oxide fuel-cell applications.
Results
eReaxFF force field validation and quality assessment
BZY20 is one of the most prominent electrolyte materials for SOEC applications22. We reproduce the following density functional theory (DFT) data with the newly developed eReaxFF force field to evaluate the quality of the eReaxFF force field and its ability to reproduce various chemical and/or electrochemical reactions in BZY20 that collectively lead to a hydrogen-generation reaction in BZY20: optimum yttrium (Y)-do** site in a multi-elemental BZY20 lattice structure, oxygen-vacancy (Ov) formation energies as a function of Ov concentration and distribution in bulk and surface regions, equation of state, Ov formation energies as a function of surface orientation and surface terminations, H2O adsorption and splitting reaction energies, excess electron localization energies, and progression of overall H2-formation-reaction without (Fig. 5) and with (Fig. 7) the presence of excess electrons (or applied electric voltage). Overall, we found good qualitative as well as quantitative agreement between DFT data and eReaxFF data as described below, and the envisaged H2-formation-reaction progression.
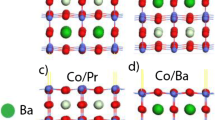
A BZY20 bulk structure configuration Type 3 (third form of Y do**) was the most energetically stable. It was consistently predicted by both eReaxFF and DFT methods (Fig. 1a). In a subsequent eReaxFF force field assessment, we created oxygen vacancies in a bulk BZY20 material by removing one or two oxygen atoms at different lattice sites, as shown in Fig. 1b by a hollow red sphere. The “t1 O vac” and “t2 O vac” structures represent BZY20 bulk structure with one oxygen vacancy, while “2 t1 O vac” and “2 t2 O vac” structures denote BZY20 bulk structure with two oxygen vacancies. The oxygen vacancy formation energy is calculated by the following equation,
where \({E}_{{{\mathrm{without}}\_{\mathrm{vacancy}}}}\) and \({E}_{{{\mathrm{with}}\_{\mathrm{vacancy}}}}\) represents the energies of solid oxides without and with oxygen vacancies, respectively, \({E}_{{\mathrm{O2}}}\) represents the energy of an oxygen molecule in the gas phase and n represents the number of oxygen atoms removed from the solid oxide to create oxygen vacancies. The DFT reference energy for an oxygen molecule in the gas phase is −31.86622 Hartree. The comparison of Ov formation energies calculated for these structures by DFT and eReaxFF methods (also shown in Fig. 1b) shows a similar qualitative trend. These energies are also tabulated in Supplementary Table 1. The negative number indicates that the existence of oxygen vacancies in the structures is not energetically favorable. In other words, the Ov’s at different lattice sites may exhibit different formation energies; therefore, they would have different formation and evolution dynamics. Moreover, Fig. 1c shows the equation of the state of the BZY20 bulk structure calculated with both eReaxFF and DFT methods. The two methods show excellent agreement, especially around the region of equilibrium volume. Alternately, the consistency in equilibrium volume demonstrates the consistency in equilibrium bond lengths calculated with eReaxFF and DFT methods.
a BZY20 bulk structures with different Y-do** positions and comparison of their energies calculated by both DFT and eReaxFF methods. b BZY20 third form of Y do** with different numbers and sites of oxygen vacancies (marked as red hollow circles) and comparison of their oxygen vacancy formation energies calculated by both DFT and eReaxFF methods. c DFT- and eReaxFF-method comparisons of the equation of state for BZY20 configuration Type 3.
In order to capture various crucial BZY20 surface-chemistry reaction pathways, we built the BZY20 slab models from the energetically favorable BZY20 configuration Type 3 (Fig. 2a). We considered two different surface orientations during the slab-model generation: (100) and (110) directions. We also considered two different terminations for the (100) surface: Ba-O and Zr-Y-O terminations of BZY20. Further, we created surface-oxygen vacancies at different sites with different concentrations (Fig. 2b, c) from the newly constructed slab models. Although the 12.5 and 25% oxygen vacancies were much higher than in real-life applications, it was convenient for us to remove multiples of 12.5% oxygen to create vacancies based on the size of the unit cell used for the DFT calculations. Our goal was to create a force field that describes oxygen vacancies and their role in creating active sites for water molecule adsorption and water splitting. Following the similar approach in eq1, we calculated and compared the corresponding surface oxygen-vacancy formation energies based on eReaxFF and DFT methods and summarized them in Supplementary Table 2. Akin to the bulk structure findings, the vacancy formation energies for slab models are also negative, meaning that the presence of vacancy is not energetically favorable, and Ov formation and evolution can change from lattice site to lattice site. For our force field fitting, we prioritized more on the energy differences for different Y-do** sites, bond lengths, and equation of states of the solid oxide to ensure equilibrium bond lengths and volume were well reproduced. We did not focus strongly on fitting the absolute values of the bulk and surface oxygen vacancy formation energies, rather focused on the qualitative trends that are shown in Supplementary Tables 1, 2. This at least ensured we can simulate the reaction dynamics qualitatively. However, a better agreement between eReaxFF and DFT values is probably achievable by further refitting the force field.
a Top view of BZY20 slab models with different surface orientations and surface terminations. b Top view of BZY20 (100) surfaces with different terminations and surface-oxygen-vacancy (Ov) concentrations and sites. c Top view of BZY20 (110) surfaces with Ba-Zr-Y-O termination and different surface-oxygen-vacancy (Ov) concentrations and sites.
We also calculated reaction energies for H2O gas adsorption and H2O splitting to H and OH, where OH would locate at a surface-oxygen-vacancy site and H would connect to a lattice oxygen. Fig. 3a shows an H2O gas adsorption and splitting reaction with energies calculated by eReaxFF and DFT for (100) surface with Zr-Y-O termination with different oxygen vacancy concentrations. Fig. 3b shows the H2-gas-generation reaction energies calculated by eReaxFF and DFT for the same surface. The H2O gas adsorption and splitting reaction energies for the (100) surface with Ba-O termination and (110) surface with Ba-Zr-Y-O termination is reported in Supplementary Table 3. The H2O gas adsorption and splitting reactions are less favorable (more endothermic) on the Ba-O terminated (100) surface than on the Zr-Y-O terminated (100) surface. The reaction energies are calculated by the differences in energies between the product and the reactants. The DFT reference energies for H2O in the gas phase and H2 in the gas phase are −17.22 Hartree and −1.166 Hartree, respectively. The eReaxFF calculated energies agree quite well with the DFT energies, indicating that the newly developed eReaxFF force field has been trained well to reliably reproduce the DFT data.
Finally, to train the excess electron interactions in eReaxFF, we attempted to reproduce constrained density functional theory (C-DFT) data corresponding to an excess electron constrained near different oxygen sites of an isolated BZY20 cluster. eReaxFF can qualitatively reproduce the relative differences in energy, as shown in Fig. 4. The most stable configuration, according to both eReaxFF and C-DFT, is found to be the one in which the excess electron is constrained on an oxygen atom on the Ba-O terminated surface and above yttrium (oxygen site O9, as shown in Fig. 4). Also, as shown in Supplementary Fig. 1, the electron-transition barrier from one oxygen site (O1) to another (O10) has been parameterized to be relatively low (16.87 kcal/mol) such that we can expect electrons to easily move from site to site at the simulation temperature. We have also parameterized the force field for the electron constrained at different oxygen sites of an isolated BZY20 cluster with hydrogenated surface (Supplementary Fig. 2) as well as for a low electron-transition barrier (9.85 kcal/mol, Supplementary Fig. 3). Based on the above discussion, we can reemphasize that our force field is capable of quantitively and quantitatively reproducing the relevant DFT and C-DFT data very well.
Using our newly developed eReaxFF force field, we performed simulations on a BZY20 slab model with a Zr-Y-O terminated surface with 12.5% surface oxygen vacancies to understand hydrogen formation reaction with the adsorption of water molecules. The oxygen vacancies are located between the Zr and Y atoms. The progression of water adsorption on the oxygen vacancy site and the eventual formation of hydrogen gas is envisaged as follows (Kröger–Vink notations for effective charges: ×, •, and ‘represents neutral, single positive, and single negative charged states, respectively):
where V••O and O×O are oxygen vacancy at the oxygen lattice site and oxygen atom at the oxygen lattice site with effective double positive and neutral charges, respectively.
The successful validation of this reaction sets the stage to perform relatively larger length-scale and longer timescale simulations in BZY20 material. The simulation progresses as follows: an H2O molecule adsorbs at the oxygen vacancy site and splits into an H and an OH. H adsorbs on a lattice O while OH settles at the Ov site. Subsequently, the two OH splits and the two H, with two excess electrons possibly emerging from applied external voltage, bind to form H2 gas. In eReaxFF, the ACKS2 charge-calculation scheme will evaluate the charges of every atom, and this will be updated based on the atomic positions as well as proximity to explicit electrons.
In order to determine the binding energy of the water molecule and the energy barriers concerning water-splitting and hydrogen-generation reactions, we performed bond scans using a biasing potential described in the Methods section. Figure 5 shows the different stages and energy barriers of the reactions. The biasing potential (described in the Methods section) is subtracted from the potential energy shown.
From Fig. 5a, it can be seen that water, initially far above the surface (Stage 1), finds the oxygen-vacancy site and adsorbs with an adsorption energy of 65 kcal/mol (Stage 2). Now the water can split into H and OH where OH is situated at the Ov site, and H can choose lattice oxygen either between a Y and Zr (Path A) (Stage 3b), or between two Zr (Path B) (Stage 3a). Path A is more favorable because it has a lower energy barrier (7 kcal /mol) than Path B’s (9 kcal/mol). Also, Path A is more exothermic (−16 kcal/mol) than Path B (−11 kcal/mol). The H dissociation from OH and eventual H2 formation can occur either from Stage 2 or Stage 3b, as shown in Fig. 5b. H2 generation directly from Stage 2 is more favorable than from Stage 3b, but for both cases, the energy barriers are extremely high (70 and 90 kcal/mol, respectively) and endothermic, indicating that these reactions might not occur in the solid-oxide operating temperature of 800 K.
On the surface with Ba-O termination, on the other hand, the adsorption energy of a water molecule to the oxygen-vacancy site (OvY; that is, Ov on top of Y) is 91.1 kcal/mol, which is more stable than the adsorption on the Zr-Y-O termination (65 kcal/mol). The pathways for water-splitting reactions on the Ba-O terminated surface are depicted in Fig. 6. Unlike the Zr-Y-O surface, the water-splitting reaction on the oxygen vacancy is required to overcome a higher energy barrier (37.5 kcal/mol) with slightly endothermic reaction energy (3.5 kcal/mol). Thus, a single water molecule will not easily dissociate on the vacancy site. However, when an extra water molecule assists a proton transfer from the bound water to the surface oxygen (the catalytic assistance of water molecule), it significantly lowers the energy barrier, to 13.4 kcal/mol—about one-third of the energy barrier for the single water reaction—because of the easier H-transfer through the H bond network. Water splitting to the subsurface layer is also endothermic, with an energy barrier of 32.8 kcal/mol, indicating that the proton diffusion to subsurface layers rarely occurs at low temperatures. Overall, the water-splitting reaction on the vacancy site of the Ba-O termination is both thermodynamically and kinetically less favorable than that on the Zr-Y-O termination.
For both cases, no excess electrons, thus no applied voltage, are present in the solid oxide. It is expected that at applied voltages, the H2 generation barrier might get lowered and H2 generation might easily occur in the simulations. Figure 7 shows the energy profiles (see purple curves) for H2 generation on the Ba-O terminated surface without (Fig. 7a) and with (Fig. 7b) the presence of an excess electron. The energy barrier without excess electron is 67 kcal/mol, whereas the presence of an excess electron on the oxygen atom of one of the reacting OH (Fig. 7c) over-coordinates the oxygen atom and consequently elongates and cleaves the corresponding OH bond. The dissociated H atom of the cleaved OH bond finds the nearby H atom of the other reacting OH, which also elongates and dissociates at this point, and finally H–H bond forms to generate H2 with a relatively lower energy barrier (45 kcal/mol). Even with the presence of excess electrons, this energy barrier is quite high to be observed in MD simulations within the simulation run time. To accelerate the H2 generation, we applied a bias in the force field to strengthen the H–H bond. The H–H bond parameters that were changed to reflect this bias are shown in Supplementary Table 4. Using the parameters corresponding to the biased H–H bond, the energy barrier for H2 generation goes down to 28.4 kcal/mol for the case without the presence of excess electrons (green curve in Fig. 7a) whereas the barrier goes down to 20 kcal/mol for the case with the presence of an excess electron (green curve in Fig. 7b). Now it can be expected that H2 generation will occur with the presence of applied voltage (simulated by addition of excess electrons) within the simulation timeframe.
Apart from oxygen-vacancy sites, an H2O molecule can be adsorbed on the Zr or Y site of a Zr-Y-O terminated surface. In such a scenario, the H2O adsorption energies are lower (adsorption energy on Zr and Y are 37.4 and 12 kcal/mol, respectively) than the case of adsorption on the vacancy sites (65 kcal/mol); however, the OH splitting energy barriers are relatively lower (Supplementary Fig. 4). Besides, we observed a similar evolution of H2O molecules where H2O splits into OH and H, irrespective of the H2O landing site. Also, the newly formed H migrates to O atoms from the surface first, before continuing its subsequent migration on the surface or to the bulk BZY20 structure. We described additional bond-restrained simulations for proton transfer on both the surface and subsurface regions in Supplementary Figs. 5, 6.
Hydrogen formation without applied voltage (excess electrons absent)
The eReaxFF force field validation described in the previous section sets the stage to perform a larger simulation of BZY20 material and delve into the key mechanisms that lead to hydrogen formation. Here we explore a system made up of steam (or H2O molecules) exposed to (100) surface of BZY20 solid oxide with Zr-Y-O termination and with 12.5% oxygen vacancy between Zr and Y atoms. Whereas various reaction-evolution scenarios are plausible, we will focus on one of the reaction-evolution pathways to demonstrate the applicability of the newly developed force field under a wide range of material and operational characteristics. We used a periodic simulation box of 42.95 Å × 42.90 Å × 50.00 Å. The amount of vacuum above the (100) surface corresponds to a length of 23.00 Å in the z-direction. We generated 25 vacancy sites on the (100) BZY20 surface with Zr-Y-O termination, which corresponds to 12.5% surface oxygen vacancy. We used 10 H2O molecules to simulate superheated steam on a BZY20 surface that corresponds to a density of 0.0065 g/cm3. The density of actual superheated steam at 773 K is 0.00028 g/cm3; however, in our simulation, we used higher steam density to include enough water molecules for the investigation than would otherwise have been possible. An actual fuel electrode interface will also be exposed to both H2 and H2O. Although we have not simulated the actual fuel electrode conditions, including both H2 and H2O molecules, our target for this stage of the force field development process was to show the method’s capability in simulating H2O adsorption, splitting, proton transfer, etc. Further, we used a temperature of 1000 K in order to enhance the reaction dynamics over the molecular dynamics (MD) simulation time we investigated. A 200 ps simulation of a system with 3000 atoms took 48 h of CPU time when four CPUs were utilized. Zero applied voltage (excess electrons absent) simulations were performed using the Amsterdam modeling suite (AMS)23.
We performed MD constant number of molecules, volume, and temperature (NVT) ensemble simulations with an intermittent flow of steam to observe the saturation of H2O adsorption on the BZY20 surface. We added 10 H2O molecules every 200 ps because we observed that it takes almost 200 ps for all 10 H2O molecules to adsorb on the surface. The H2O molecules were introduced frequently to accelerate the targeted events in the MD simulations. Figure 8a shows the snapshot at the beginning of the simulation. Throughout the course of the MD simulation, after the first installment of 10 H2O molecules, all but one H2O molecule adsorbs at the vacancy sites, and the remaining one adsorbs at Zr. All adsorbed H2O molecules split into H and OH, and H moves to lattice oxygen sites between a Zr and a Y atom, which are favorable sites compared to the lattice oxygen sites between two Zr atoms. Three protons transfer to subsurface oxygen atoms, among them, two transfer to the second from the top layer and one transfer to the third layer. This proves the current force field’s capability of simulating proton transfer to bulk regions of the solid oxide from the surface. After a total of 70 H2O molecules were introduced, all but 2 H2O molecules were adsorbed, as shown in the snapshot at the end of the 1.4 ns simulation in Fig. 8b. All the vacancy sites were filled, and the remaining H2O molecules were adsorbed either on Y or Zr. 64% of the surface Y and 36% of the surface Zr were bound to H2O. All adsorbed H2O split into H and OH. Proton transfer to the sixth layer from the top surface was observed (Fig. 8b). This was a zero-voltage simulation, so as expected, no H2 generation was observed due to the extremely high energy barrier associated with this reaction. With the application of external voltage (or excess electron presence), H2 generation can be expected.
In contrast, as seen in Fig. 9a, only 41% of water molecules (45 H2O) split into H and OH on the Ba-O terminated surface with 12.5% Ov after 11 cycles of an intermittent flow of steam. Due to the higher energy barrier and the higher reaction energy, the water-splitting reaction takes place slower than it would on the Zr-Y-O terminated surface. 47% of the total surface oxygen sites were converted into hydroxyl groups. The remaining 65 H2O molecules formed a thin layer near the surface. Eight protons were transferred to the subsurface or deeper layer of oxygen, and the deepest proton transfer was observed in the fourth layer from the top surface. No H2 generation was observed. On the defect-free surface (Fig. 9b), the water-splitting reaction was faster than the surface with O vacancy (Fig. 9c). After 11 cycles, 57% of the surface oxygen sites were converted into hydroxyl groups. By tracking the reactions occurring in the MD simulations, it was observed that unlike the reaction observed in the Ov-defect surface, a gas-phase water molecule directly reacted with the surface oxygen to transfer a proton, and the hydroxyl group of the dissociated water was bound to the neighboring Ba. This hydroxyl group can diffuse fast from one Ba site to the other on the surface (Fig. 10). The potential-energy profile of the water dissociation on the defect-free surface (Fig. 10c) indicates that this reaction is spontaneous with the reaction energy of −4 kcal/mol. Because the water-splitting reaction along this pathway is thermodynamically and kinetically more favorable than the pathways described in Fig. 6, this was the predominant mechanism on the Ba-O terminated surface. However, the Zr-Y-O terminated surface was still more active for water dissociation than the Ba-O terminated surface because the oxygen density on the surface is higher, indicating that more oxygen vacancy sites were accessible to water.
In addition to the previous simulations for surface vacancies, we performed simulations with oxygen vacancies in the bulk to observe the effects of temperature on surface-vacancy occupation, probable H2 formation, and proton transfer on the surface to the subsurface and bulk regions. Three BZY20 structures, having bulk-oxygen vacancies (Ov), 0, 12.5, and 25%, were created. Each Ov model was then subjected to relaxation simulation using a fixed volume and temperature ensemble (NVT) at three temperatures: 800, 1000, and 1200 K. Initially, 40 water molecules were introduced above the Zr-Y-O terminated surface (Fig. 11), and the simulation was run for 500 ps. This procedure was followed until a total of 160 water molecules were introduced over a period of 2 ns.
Figure 12 shows the vacancy occupation as a function of simulation time. The total number of Ov in 12.5 and 25% of models on the surface layer is 28 and 48, respectively. An increase in occupation of the Ov sites is observed with time. This is because after every 500 ps, 40 water molecules were added, which increases the number of water molecules in the system and also the adsorption on the Ov site. The increase in thermal energy (i.e., rise in temperature) helps in overcoming the high energy barrier and thus results in more filling of Ov at higher temperatures.
Because the energy barrier for H2 generation at zero applied voltage is extremely high, it would take an extremely long simulation time to observe H2 formation. Instead, we looked into the possible number of H2 formation sites by counting the instances when the H–H distance becomes less than 4 Å, as shown in Fig. 13. Figure 14 shows the possible number of H2 formation sites as a function of the simulation time. The 12.5% Ov model (run at 800 K) shows zero possibility of H2 formation because less than 20% of Ov are occupied. This results in a decrease in the probability of possible H2 formation. The 25% Ov model (run at 1200 K) shows the enhanced possibility of H2 formation as more than 70% of oxygen vacancies are filled, as shown in Fig. 13. Thus, the greater the number of Ov, the greater the possibility of OH− ions occupying the vacancy sites, and the higher the probability of possible H2 formation sites.
Proton migration occurs through three steps: (a) dissociative adsorption—water dissociates into OH− and H+ ions, and OH− ions gets adsorbed at Ov site, (b) Grotthuss mechanism—H+ ions interact and form weak bonds with neighboring O atoms, and (c) rotational diffusion—the H+ ions rotate and reorient to facilitate the transport of protons from one to the neighboring oxygen atom21. Figure 15a–c show the migration of protons (H+) on the surface, subsurface, and within the bulk layers, respectively. An increase in proton migration on the surface layer as a function of time is observed for the three Ov models (Fig. 15a). This is because the number of water molecules dissociated into OH− and H+ ions increases with simulation time because 40 water molecules were introduced after every 500 ps. For each Ov model, the relative difference of proton migration between three temperatures is in the range between 0 and 9 while the difference of proton migration between the 0 and 12.5% and between the 12.5 and 25% Ov models is in the range of 30–35. This clearly shows that the amount of Ov in the model has a more significant effect than temperature. The 25% Ov model shows the highest proton migration on the surface layer. This is attributed to the fact that a greater number of Ov sites exist on the surface layer, and this increases the possibility of dissociation of more water molecules into OH− and H+ ions. A similar increasing trend was observed in the case of proton migration in the subsurface layer (Fig. 15b). However, a smaller number of migrations occurs at the subsurface than on the surface layer because the hop** mechanism in proton transfer must overcome a higher energy barrier. In addition, an even smaller number of migrations in the bulk layer (Fig. 15c) is observed due to the energy barrier for the hop** of the proton. Each Ov model at a given temperature shows 0–4 proton migrations as a function of time in the bulk layers, except for the 0% Ov model—which ran at 1200 K—where proton migration was greater due to high thermal energy available to overcome the hop** barrier and the availability of all neighboring O atoms (no defects) to form weak bonds with H+ ions. In a separate study24 we simulated the migrations of oxygen vacancies in BZY20. Vacancies tend to migrate toward the surface and these migrations are enhanced with temperature. As mentioned earlier, we are simulating our system with a higher density of steam than a real-life fuel electrode interface and adding steam frequently to enhance the dynamics. A real-life structure is likely to have some damage, dislocations, grain boundaries, different concentrations of oxygen vacancies than we simulated, and the presence of excess electrons as well as electronic hole defects, and these might alter the competition between electronic holes’, oxygen vacancy’s, protons’ migrations at different temperatures. In addition, the gas entropy effect in a realistic situation might alter the temperature dependence of proton concentrations.
Hydrogen formation with applied voltage (excess electrons present)
H2 formation with applied voltage was performed as follows. To simulate externally applied voltage, we randomly placed ten excess electrons near oxygen atoms in the bulk region of a BZY20 structure with Ba-O terminated surface with all surface oxygen atoms hydrogenated (Fig. 16a). We introduced an excess charge to the system to resemble an externally applied voltage. Following a similar approach as the work done by Zheng and Balbuena25 and our previous work26, we emulated external voltage by adding excess electrons to the system. The electrons transition from one oxygen site to another throughout the simulation. After 8 ps of MD simulation at 1000 K, four out of ten excess electrons diffused, reached the surface, and acted as potential H2 formation sites (Fig. 16b). The electrons diffused in such a way that they chose O atoms that were connected to Y atoms. Fig. 17 shows the total path traveled by the excess electrons in 202 ps of MD simulation. Throughout the 202 ps simulation, one H2 molecule formed at 180 ps. As described earlier, the excess electron elongated and split the OH bond, and the dissociated H atom found a nearby OH group and, eventually, H2 formed. The snapshots of the steps leading to the H2 formation are shown in Supplementary Fig. 7. After H2 formed, the associated excess electron remained on the dissociated O atom for the rest of the simulation time, as shown in Supplementary Fig. 8. Three other excess electrons remained on the OH sites (Supplementary Fig. 8) and it was expected that three more H2 could form if the simulation was run longer. The generated H2 molecule is seen floating above the surface in Fig. 16c.
Additionally, we performed the above same simulation at 1200 K and observed that four out of ten excess electrons diffuse to the surface at 7 ps, H2 molecules from three of the four possible sites form over the 198.5 ps MD simulation as shown in Supplementary Fig. 9. Although we biased the H–H bond strength to accelerate H2-formation dynamics, H2 cannot form without excess voltage—in other words, excess electrons. To prove this, we performed the same simulation for 250 ps, using the biased force field parameters at both 1000 and 1200 K without excess electrons, and observed that no H2 formed. These non-zero applied voltage (excess electrons present) simulations were performed using the serial version of the eReaxFF code.
Discussion
Many fundamental electrochemical processes in SOECs are still elusive and need detailed theoretical insights into the various intricate electrochemical processes that lead to efficient H2 formation and overcome existing experimental limitations, including reliable fundamental length-scale and timescale access. For instance, detailed mechanisms of the intricate interfacial physical and chemical phenomena in solid-oxide electrolysis devices have not been explored comprehensively and remained unclear despite significant experimental efforts and theoretical advances27,28,29,30. The atomistic simulations can effectively enlighten the issues pertinent to electrolytic device chemistry and the change in interface properties during operation. Modeling techniques can help elucidate the complexities of catalyst and electrode/electrolyte interface at the atomic and molecular levels while providing insights into the depictions of the solvent, cations, and anions near the interface, as well as the mechanisms and reaction barriers of key steps involving proton/electron transfers in any reaction environment—lenient to harsh. Modeling the electrocatalytic process required to describe bond-breaking and bond-making processes make a quantum chemical treatment, such as DFT, useful. Various applications of DFT are detailed somewhere else9. However, the inability of DFT to simulate large length-scales and long timescales limits their application. Fortunately, empirical force field-based methods, such as reactive force fields, can simulate reactions at larger lengths and time scales.
The ReaxFF reactive force field method has been employed in many chemical systems, including solid-oxide fuel cells31. However, the ReaxFF method is limited as far as an investigation of electron conductivity and electrochemical phenomena relevant to electron migration and interactions are concerned. A recent advancement in ReaxFF methodology, such as the eReaxFF method32,33 with a description of an explicit pseudo-classical electron-like particle, can address such fundamental research needs. Recently, the eReaxFF method has been applied to simulate electronic motion in carbon-based32 and lithium-electrolyte systems34 and was recently extended to Ag-metal systems35, polyethylene electrical breakdown36, acetophenone radical37 and graphitic anodes of lithium-ion batteries26. No other empirical force field-based methods have been reported in the literature for a thorough investigation of various intricate chemical and electrochemical processes encountered in solid oxide electrocatalysis, probably due to the complexity (e.g., availability of reliable basis sets) associated with multi-elemental system and their interactions in solid oxides and also in electrocatalysis process itself. The developed eReaxFF force field is capable of simulating electronic motion, localization, and electrochemical reactions in BZY20 solid oxide using explicit electrons in a classical framework. This enables larger length-scale and longer timescales than traditional ab initio methods. Yttrium-doped Barium Zirconate (BZY) electrolyte material, in general, exhibits a large band gap with low electronic conductivity but possesses high ionic conductivity. Co-sintering of BZY with electron-conducting catalysts such as nickel on the fuel electrode side would be important to facilitate surface electrocatalytic reactions, e.g., H2O dissociation and H2 formation. Researchers often use the same proton-conducting material for proton-conducting SOECs, which limits the improvement of the performance38. Using a BZCY-Ni fuel electrode and BZY electrolyte, ref. 39 confirmed improved proton conductivity, electrochemical performance, stability, Faradaic efficiency, and energy efficiency. Cermet electrodes, such as composites consisting of Ni and BCZY, serve as catalysts and have shown satisfactory electrochemical performance, stability, high conductivity in reducing environments, and excellent catalytic activity40,41. As a future direction, our method can be extended to include the possibly altered reaction mechanisms that may occur with the presence of electrocatalysts. The oxidation of oxygen vacancies creates electronic defects such as hole defects42. The increased oxygen chemical potential in the air-electrode side will increase hole conduction and decrease faradaic and energy efficiency22,43. The all-electron quasi-Drude version of eReaxFF that we recently developed for Ag-metal35 and graphitic26 systems described every neutral atom of the system as a cation paired with an explicit electron. To describe the electron delocalization of metals or the aromatic delocalization of graphene, we employed the Drude-like eReaxFF model so that electrons can easily dissociate from the host atom as well as couple back into the over-coordination term of the host atoms. We simulated both charge-neutral systems as well as charged systems with excess electrons and the quasi-Drude model helped us eliminate the ACKS2 charge calculation altogether, saving tremendous simulation run time. For the present model, we have not used the all-electron quasi-Drude approach, but extending the model to describe atom types in a quasi-Drude fashion would allow the description of electronic holes in a very natural way—by simply removing an electron. This will enable the study of hole conduction in the air-electrode side in a more physically correct way than having an explicit hole-like particle description. Further development can enable electronic-leakage simulations, defect-defect interactions, and defect-dopant interactions in addition to other relevant solid-oxide phenomena. With everything in place, a demonstration of a large-scale simulation, consisting of a fuel electrode with actual fuel electrode conditions including both H2 and H2O molecules, electrolyte as well as an air-electrode, capable of elucidating the intricate mechanisms and kinetics governing hydrogen-evolution reactions is plausible. Besides, the eReaxFF force field development approach and applications presented in the current investigation can be extended to address similar issues in other renewable-energy applications (e.g., ionic conductivity in solid-state lithium batteries44, as well as to understand the delicate process of lithium electrodeposition that can dictate the lithium-metal battery performance45) where electrons play a pivotal role in the complex multi-elemental and multi-component environment.
While emphasizing the capabilities and applications of the eReaxFF approach, we also like to highlight various potential limitations of the eReaxFF method. First, the capability of the eReaxFF force field is dependent on the quality and quantity of experimental and quantum-chemistry data used to parameterize the eReaxFF force field. Our DFT calculations were performed using the PBE-GGA functional. The underestimation of band gap is a known issue for calculations at the GGA level and this may cause error in the defect state levels within the band gap. More comprehensive and accurate experimental and quantum-chemistry data would result in a more accurate description of the eReaxFF force field. Second, the existing DFT codes may have limitations in allowing explicit-electron treatment. For instance, our C-DFT calculations did not allow us to simulate periodic boundary conditions due to the limited theoretical advances (e.g., availability of high-quality basis sets) in modeling periodic multi-element systems like BZY20; hence, the calculations were performed on isolated slabs with limited system size. Also, not many basis sets had Ba included, and performing an all-electron basis set was not possible due to the computationally intensive and time-consuming steps in C-DFT. Besides, the targets for training electron-transition barriers had to be assumed because no known reliable computational or experimental method was available to obtain data related to electron transition from one atom site to another. Our targets were set in such a way as to allow for easy electron transitions in the operating temperature of the solid oxide. Finally, we applied a bias to the H–H bond to allow for faster H2 formations in the timescale we used for simulations, as the exact timescale on which a reliable H2 formation occurs in typical experimental studies was unknown. The future developments in theoretical methods such as C-DFT are likely to address the current limitations of the eReaxFF method and make it more practical in the simulation of complex fundamental chemical and electrochemical processes encountered in a plethora of renewable-energy applications.
As concluding remarks, we have successfully developed an eReaxFF force field and demonstrated the relevant molecular dynamics simulation approach in large length-scale, and longer timescale atomistic simulations of a comprehensive hydrogen generation reaction in BaZr0.8Y0.2O3-δ (BZY20) solid-oxide material. The newly developed eReaxFF force field reproduces quantum-mechanical data very well. The zero-voltage (excess electrons absent) simulations of steam adsorption on the BZY20 surface with oxygen vacancies concluded that hydrogen generation does not occur at an operating temperature of 1000 K. Further, the introduction of explicit-electron concept by parameterizing the eReaxFF with C-DFT data enabled the non-zero-voltage simulation of hydrogen generation and set the stage to simulate complex electron conductivity, electron leakage, and other non-zero-voltage phenomena in solid oxide electrolysis cells. Besides, the eReaxFF force field can be extended to include additional dopants (elemental compositions) to show the effects on oxygen migrations and hydrogen generation in solid oxide electrolysis cells. Finally, the eReaxFF force field-based atomistic-simulation method could be a promising tool in unlocking a fundamental understanding of other renewable-energy applications, such as lithium-metal and solid-state batteries.
Methods
Density functional theory (DFT)
We generated a suite of condensed phase DFT data that describe the BZY20 bulk and slab material structures, with and without the presence of oxygen vacancies, and with water-adsorption energies, water splitting, and hydrogen-generation energies in (100) and (110) surfaces with Ba-O and Zr-Y-O surface terminations. We considered various possible configurations for each case, including the pristine structures of BZY20 as well as BZY20 structures with oxygen vacancies at different locations and concentrations. We performed similar calculations in the case of slab models with different concentrations of hydrogenation. Based on these comprehensive DFT calculations, we obtained oxygen migration, water adsorption, and hydrogen generation energies. We subsequently used all these data in an eReaxFF force field training process. Next, these generated DFT datasets were subsequently added to a previously generated ReaxFF training data set for YSZ solid oxide31 to parameterize the eReaxFF Ba/Zr/Y/O atom, bond, angle, and dihedral parameters, aiming to optimally reproduce the generated DFT data and make our eReaxFF force field training set more comprehensive. We used a combination of VASP, CP2K plane-wave methods, and PBE functionals to generate the condensed-phase DFT data required for the purported force field training.
Constrained density functional theory (C-DFT)
Training the eReaxFF force field against electronic structures determined from DFT alone can be complicated, especially for energy-value calculations for a geometry-optimized structure with an excess electron constrained at different lattice positions. Consequently, we used C-DFT46 to incorporate electron-localization behavior in our eReaxFF description and to train our force field. The C-DFT method allowed the constraining of excess electronic charge to a specific region of the BZY20 structure. A user-specified constraint can be imposed in the C-DFT method, and the total energy can be minimized by adding a Lagrange multiplier to the total energy functional as shown below37,46:
where, E(N) is the total energy C-DFT energy, \(E\left[\rho \left(r\right)\right]\) is the standard DFT energy, λ is a Lagrange proportionality constant, and N is the number of electrons to be constrained. Upon locating critical points where the gradients of the function and constraint are proportional, the lowest energy state that also satisfies the constraint is chosen37,46,47. The relative energy values can be used to train the eReaxFF force field—thus yielding important details about the structure’s energetically ideal sites for an excess electron. For convenience, we used single-atom constraints for our eReaxFF development. Although it is anticipated that single-atom constraints will result in artificially high C-DFT energies46, the relative energy estimates corresponding to single-atom constraints should continue to be directly comparable.
We have performed C-DFT energy minimizations on isolated BZY20 structures in the gas phase with an excess electron constrained at different oxygen atoms because oxygen is the most electronegative among other elements of the BZY20, and excess electron is most likely to localize near oxygen sites. The C-DFT calculations were performed using the NWChem software48 with molecular charge and spin constrained to −1 and +1, respectively. The geometry was optimized using the B3LYP functional with the 3–21G basis set for all atoms except Ba, for which we used the wtbs basis set. Finally, we demonstrate the quality of the newly developed force field in simulating water adsorption, water splitting, and eventual proton transfer and hydrogen generation at zero-voltage of the BZY20 solid oxide. The introduction of the explicit-electron concept enables non-zero-voltage effects on hydrogen formation. Quality of the force field trends opens up an avenue for further training the force field for the purpose of simulating various other non-zero-voltage electrochemical processes in SOEC applications, including electron conductivity, electron leakage, etc. with explicit electrons.
eReaxFF force field details
eReaxFF method is an extension of the standard ReaxFF method that incorporates a pseudo-classical explicit-electron scheme. The general expression of the eReaxFF energy is
where partial-energy contributions include bond, over-coordination penalty, and under-coordination stability, lone pair, valence, torsion, nonbonded van der Waals, Coulomb, electron–nucleus interactions, and electron–electron interactions, respectively. All the many-body bonded interaction and nonbonded interaction terms of ReaxFF are retained with modifications made to the over-coordination penalty, under-coordination stability, and lone pair terms to incorporate explicit electrons. In addition, new energy functionals are included to account for electron–nucleus and electron–electron interactions.
In ReaxFF, the bonded and nonbonded interactions are calculated independently; that is, the valency and number of lone pair electrons of an atom type are independent of atomic charge. Thus, the bond orders are fully decoupled from the charges; for this reason, a hydrogen ion (H+) is considered capable of forming bonds. eReaxFF, on the other hand, fixes this shortcoming by introducing variable valency and the number of lone-pair electrons of an atom depending on the proximity of explicit electrons. The bond orders and over-coordination, under-coordination, and lone pair energies are calculated based on corrected valence electrons. Removing an electron from a hydrogen atom, for instance, decreases its valency and, consequently, increases the over-coordination of an existing bond, resulting in a larger over-coordination energy penalty. It thereby reduces the bond order associated with that atom and weakens the bond. The effect of the proximity of explicit electrons is described by each atom type’s own set of rules defined in eReaxFF. In contrast to ReaxFF, this scheme has enabled the eReaxFF method to counteract the formation of unphysical bonds such as H+–H+ due to the higher over-coordination penalty stemming from the loss of valence electrons from the hydrogen atoms. To compute atomic charges, eReaxFF uses the atom-condensed Kohn–Sham DFT approximated to the second order (ACKS2) charge-calculation scheme, which eliminates long-range charge smearing, in contrast to the electronegativity equalization method (EEM) or the charge equilibrium method (QEq) used by most ReaxFF force fields. The ACKS2 charge-calculation scheme provides non-integer numbers for partial atomic charges, and the implicit treatment of electrons, alone, cannot distinguish between radicals and ions very well. The proximity of explicit electron or explicit hole changes the partial atomic charges due to the charge-valency coupling of eReaxFF. The summation of an atom’s ACKS2 calculated charge and the charge of the explicit electron or hole (i.e., −1 or +1, respectively) that resides on that atom will define the total charge of that atom. The presence or absence of an explicit electron or hole can differentiate between hydrogen atoms and protons or between hydroxyl ions and hydroxyl radicals. The explicit electrons are treated like atoms with their own set of Cartesian coordinates. In the current eReaxFF force fields, the mass of the electron particles is considered 1 amu in order to allow 1 fs MD timesteps.
eReaxFF force field training
The eReaxFF force field parameters were developed and trained with the aim of reproducing quantum chemistry-based DFT data. Parameter optimization was conducted using a successive one-parameter search technique49 to minimize the following expression for the error:
where \({x}_{i,{\mathrm{lit}} }\) and \({x}_{i,{\mathrm{eReaxFF}} }\) are the target quantum-chemistry/experimental data and eReaxFF values of the ith entry of the force field training set and \({\sigma }_{i}\) is the inverse weight that determines how accurately that particular data needs to be fitted with the QC. The summation of all errors in the training set provides the overall error, which is one of the measures of the quality of the force field.
Biasing potential
In order to determine the binding energy of the water molecule and the energy barriers concerning water-splitting and hydrogen-generation reactions, we performed bond scans. Reaction pathways are enforced by putting a biasing potential between the two target atom pairs to move the reaction along the desired reaction coordinates. Reaction-energy barriers are computed using these reaction pathways. This additional biasing potential is introduced to the system that is intended to stretch or compress bonds at a predefined rate in order to provide the system with additional energy that can be used to overcome the reaction barrier. The biasing potential has the following form,
where f1 and f2 are force constants, and rij is the distance between the atom pair of interest and is varied along the reaction coordinate.
Data availability
The data will be provided upon reasonable request.
Code availability
The code will be provided upon reasonable request.
References
Li, R. & Li, C. In Advances in Catalysis (ed. Song, C.) Ch. 1 (Academic Press, 2017).
Majumdar, P., Bera, M. K., Pant, D. & Patra, S. Enzymatic Electrocatalysis of CO2 Reduction. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry (ed. Wandelt, K.) (Elsevier, 2018).
Groß, A. Computational modeling of electrocatalytic reactions. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry (ed. Wandelt, K.) (Elsevier, 2018).
Buttler, A. & Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review. Renew. Sustain. Energy Rev. 82, 2440–2454 (2018).
Carmo, M. & Stolten, D. In Science and Engineering of Hydrogen-Based Energy Technologies (ed. de Miranda, P. E. V.) Ch. 4 (Elsevier, 2019).
Kumar, S. S. & Himabindu, V. Hydrogen production by PEM water electrolysis–A review. Mater. Sci. Energy Technol. 2, 442–454 (2019).
Jiao, Y., Zheng, Y., Jaroniec, M. & Qiao, S. Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015).
Benck, J. D., Hellstern, T. R., Kibsgaard, J., Chakthranont, P. & Jaramillo, T. F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal. 4, 3957–3971 (2014).
Maheshwari, S., Li, Y., Agrawal, N. & Janik, M. J. In Advances in Catalysis (ed. Song, C.) Ch. 3 (Academic Press, 2018).
Sapountzi, F. M., Gracia, J. M., Fredriksson, H. O. A. & Niemantsverdriet, J. W. H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 58, 1–35 (2017).
Mahmood, N. et al. Electrocatalysts for hydrogen evolution in alkaline electrolytes: mechanisms, challenges, and prospective solutions. Adv. Sci. 5, 1700464 (2018).
Sampangi, S. K. & Lim, H. Recent advances of hydrogen production through proton exchange membrane water electrolysis–A review. Sustain. Energy Fuels 7, 3560–3583 (2023).
Kamlungsua, K., Su, P. & Chan, S. H. Hydrogen generation using solid oxide electrolysis cells. Fuel Cells 20, 644–649 (2020).
Deka, D. J. et al. Hydrogen production from water in a solid oxide electrolysis cell: effect of Ni do** on lanthanum strontium ferrite perovskite cathodes. Ind. Eng. Chem. Res 58, 22497–22505 (2019).
Fabbri, E., Pergolesi, D., Licoccia, S. & Traversa, E. Does the increase in Y-dopant concentration improve the proton conductivity of BaZr1− xYxO3− δ fuel cell electrolytes? Solid State Ion. 181, 1043–1051 (2010).
Bae, K. et al. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat. Commun. 8, 14553 (2017).
Vøllestad, E. et al. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 18, 752–759 (2019).
Schiller, G. et al. Solar heat integrated solid oxide steam electrolysis for highly efficient hydrogen production. J. Power Sources 416, 72–78 (2019).
Posdziech, O., Geißler, T., Schwarze, K. & Blumentritt, R. System development and demonstration of large-scale high-temperature electrolysis. ECS Trans. 91, 2537 (2019).
Peters, R. et al. Long-term experience with a 5/15kW-class reversible solid oxide cell system. J. Electrochem Soc. 168, 014508 (2021).
Gaikwad, P. S., Mondal, K., Shin, Y. K., van Duin, A. C. T. & Pawar, G. Enhancing the Faradaic efficiency of solid oxide electrolysis cells: progress and perspective. NPJ Comput. Mater. 9, 149 (2023).
Han, D., Toyoura, K. & Uda, T. Protonated BaZr0. 8Y0. 2O3− δ: impact of hydration on electrochemical conductivity and local crystal structure. ACS Appl Energy Mater. 4, 1666–1676 (2021).
ReaxFF 2020.103, SCM, Theoretical Chemistry, Vrije Universiteit. http://www.scm.com (2020).
Gaikwad, P., Pawar, G., Shin, Y. K., Hossain, M. J. & van Duin, A. Modeling dynamic evolution of oxygen vacancies in solid oxide materials. J. Electrochem. Soc. 170, 113501 (2023).
Zheng, Y. & Balbuena, P. B. Localized high concentration electrolytes decomposition under electron-rich environments. J. Chem. Phys. 154, 104702 (2021).
Hossain, M. J., Pawar, G. & van Duin, A. Development and applications of an eReaxFF force field for graphitic anodes of lithium-ion batteries. J. Electrochem. Soc. 169, 110540 (2022).
Ryan, E. M. & Mukherjee, P. P. Mesoscale modeling in electrochemical devices—A critical perspective. Prog. Energy Combust. Sci. 71, 118–142 (2019).
Akay, Ö. et al. Electrolysis in reduced gravitational environments: current research perspectives and future applications. NPJ Microgravity 8, 56 (2022).
Wang, M. & Feng, Z. Interfacial processes in electrochemical energy systems. Chem. Commun. 57, 10453–10468 (2021).
Shi, H., Su, C., Ran, R., Cao, J. & Shao, Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells. Prog. Nat. Sci. Mater. Int. 30, 764–774 (2020).
Van Duin, A. C. T., Merinov, B. V., Jang, S. S. & Goddard, W. A. ReaxFF reactive force field for solid oxide fuel cell systems with application to oxygen ion transport in yttria-stabilized zirconia. J. Phys. Chem. A 112, 3133–3140 (2008).
Islam, M. M., Kolesov, G., Verstraelen, T., Kaxiras, E. & Van Duin, A. C. T. EReaxFF: a pseudoclassical treatment of explicit electrons within reactive force field simulations. J. Chem. Theory Comput. 12, 3463–3472 (2016).
Leven, I. et al. Recent advances for improving the accuracy, transferability, and efficiency of reactive force fields. J. Chem. Theory Comput. 17, 3237–3251 (2021).
Islam, M. M. & Van Duin, A. C. T. Reductive decomposition reactions of ethylene carbonate by explicit electron transfer from lithium: an eReaxFF molecular dynamics study. J. Phys. Chem. C. 120, 27128–27134 (2016).
Evangelisti, B., Fichthorn, K. A. & van Duin, A. C. T. Development and initial applications of an e-ReaxFF description of Ag nanoclusters. J. Chem. Phys. 153, 104106 (2020).
Akbarian, D., Ganeshan, K., Woodward, W. H. H., Moore, J. & van Duin, A. C. T. Atomistic-scale insight into the polyethylene electrical breakdown: an eReaxFF molecular dynamics study. J. Chem. Phys. 154, 24904 (2021).
Penrod, K. A. et al. Using C-DFT to develop an e-ReaxFF force field for acetophenone radical anion. J. Chem. Phys. 155, 214104 (2021).
Lei, L. et al. Progress report on proton conducting solid oxide electrolysis cells. Adv. Funct. Mater. 29, 1903805 (2019).
Lei, L. et al. Energy storage and hydrogen production by proton conducting solid oxide electrolysis cells with a novel heterogeneous design. Energy Convers. Manag. 218, 113044 (2020).
Pirou, S. et al. Planar proton-conducting ceramic cells for hydrogen extraction: mechanical properties, electrochemical performance and up-scaling. Int. J. Hydrog. Energy 47, 6745–6754 (2022).
Yang, Y. et al. An efficient and prospective self-assembled hybrid electrocatalyst for symmetrical and reversible solid oxide cells. Electrochim. Acta 362, 137171 (2020).
Vera, C. Y. R. et al. A mini-review on proton conduction of BaZrO3-based perovskite electrolytes. J. Phys. Energy 3, 032019 (2021).
Zvonareva, I., Fu, X.-Z., Medvedev, D. & Shao, Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energy Environ. Sci. 15, 439–465 (2022).
Li, J. et al. Room temperature all-solid-state lithium batteries based on a soluble organic cage ionic conductor. Nat. Commun. 13, 2031 (2022).
Wang, X. et al. Glassy Li metal anode for high-performance rechargeable Li batteries. Nat. Mater. 19, 1339–1345 (2020).
Kaduk, B., Kowalczyk, T. & Van Voorhis, T. Constrained density functional theory. Chem. Rev. 112, 321–370 (2012).
Cohen, L. W. Lagrange multipliers for functions of infinitely many variables. (1934).
Apra, E. et al. NWChem: past, present, and future. J. Chem. Phys. 152, 184102 (2020).
Van Duin, A. C. T., Baas, J. M. A. & Van De Graaf, B. Delft molecular mechanics: a new approach to hydrocarbon force fields. Inclusion of a geometry-dependent charge calculation. J. Chem. Soc. Faraday Trans. 90, 2881–2895 (1994).
Acknowledgements
This research was supported through the Idaho National Laboratory’s Laboratory Directed Research & Development Program under the US Department of Energy–Idaho Operations Office Contract DE-AC07-05ID14517. Accordingly, the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes.
Author information
Authors and Affiliations
Contributions
M.J.H., G.P., and A.C.T.v.D. conceived the idea. M.J.H. prepared the force field training set and performed a major part of the reactive molecular dynamics simulations. A.C.T.v.D. performed the force field training. M.J.H. wrote a major part of the manuscript. P.G. and Y.K.S. also performed a part of the reactive MD simulations and wrote a part of the manuscript. J.A.S., K.A.P., M.L., and Y.L. performed the DFT simulations required to train the eReaxFF force field. M.J.H., G.P., P.G., Y.K.S., and A.C.T.v.D. extensively reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hossain, M.J., Gaikwad, P., Shin, Y.K. et al. eReaxFF force field development for BaZr0.8Y0.2O3-δ solid oxide electrolysis cells applications. npj Comput Mater 10, 136 (2024). https://doi.org/10.1038/s41524-024-01268-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41524-024-01268-9
- Springer Nature Limited