Abstract
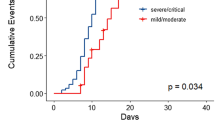
There have been reports of long coronavirus disease (long COVID) and breakthrough infections (BTIs); however, the mechanisms and pathological features of long COVID after Omicron BTIs remain unclear. Assessing long-term effects of COVID-19 and immune recovery after Omicron BTIs is crucial for understanding the disease and managing new-generation vaccines. Here, we followed up mild BA.2 BTI convalescents for six-month with routine blood tests, proteomic analysis and single-cell RNA sequencing (scRNA-seq). We found that major organs exhibited ephemeral dysfunction and recovered to normal in approximately six-month after BA.2 BTI. We also observed durable and potent levels of neutralizing antibodies against major circulating sub-variants, indicating that hybrid humoral immunity stays active. However, platelets may take longer to recover based on proteomic analyses, which also shows coagulation disorder and an imbalance between anti-pathogen immunity and metabolism six-month after BA.2 BTI. The immunity-metabolism imbalance was then confirmed with retrospective analysis of abnormal levels of hormones, low blood glucose level and coagulation profile. The long-term malfunctional coagulation and imbalance in the material metabolism and immunity may contribute to the development of long COVID and act as useful indicator for assessing recovery and the long-term impacts after Omicron sub-variant BTIs.
Similar content being viewed by others
Introduction
Over four years ago, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged, causing the coronavirus disease 2019 (COVID-19) outbreak1. Currently, the widespread variants of concern (VOCs) are derived from the Omicron sub-variant BA.2, including XBB, XBB.1.5, BQ.1, BQ.1.1, BA.5.2 and BF.7. The unusually high number of mutations in the spike (S) proteins of these variants results in a sizeable antigenic shift from previous VOCs2,3,4. While BA.5.2 and BF.7 caused widespread breakthrough infections (BTIs) in China5, XBB and XBB.1.5 infections were rapidly spreading globally and accounted for approximately 90% of the total international prevalence6. The frequent emergence of new VOCs and the gradual weakening of the vaccine-induced immunity against the prototype strain make the current vaccine strategy inadequate in protecting against VOCs with different antigenicity. Consequently, research on vaccines, antibodies and other prophylactic measures remains challenging and seriously concerning.
Meanwhile, long coronavirus disease (long COVID or Post-COVID Conditions) has attracted overwhelming global attention. It refers to a lack of return to a usual state of health following acute COVID-19 illness, including signs, symptoms, and conditions that continue or develop after acute infection, like malfunction of major organs such as the liver, kidneys and the cardiovascular system7,8. Long COVID can occur in individuals regardless of vaccination status, symptom presentation, or infection with the wild-type strain, as determined primarily through questionnaires8,9. Omicron infections result in fewer hospitalizations, less severe illness, and a higher rate of asymptomatic cases, making it challenging to evaluate the related long COVID10,11,12. A recently published study showed that approximately 70% of Omicron BA.2 related long COVID will recover in one year after infection13. However, little is known about its mechanism and possible hidden pathological features.
It has been reported that the severity of long COVID is negatively correlated with vaccination status14,13,43,Single-cell data analysis Single-cell data were integrated and clustered using the Seurat R package (version 4) (https://satijalab.org/seurat/). A total of 124,541 cells were obtained from single-cell sequencing of the nine samples, and 108,306 cells remained after quality control. The cell quality control was conducted as follows: cells with a mitochondrial gene ratio exceeding 10% were removed, and only cells with gene numbers ranging from 500 to 4500 and UMI numbers ranging from 800 to 16,000 were retained. DoubletFinder R package (https://github.com/chris-mcginnis-ucsf/DoubletFinder) was used to remove potential doublets, and further manually remove potentially marginalized doublets based on known classic markers. The filtered data were then standardized and normalized, and principal component analysis was performed on the top 2,000 genes with the highest coefficients of variation. The Harmony R package (https://github.com/immunogenomics/harmony) and the anchor module of Seurat were used to remove inter-batch effects between the samples and groups for cell clustering. Based on the elbow point and significance of the different principal components, the top 30 PCs were selected for subsequent cell clustering, and different resolutions were set to determine the cell clusters. Dimensionality reduction and visualization of single cells were performed using the Uniform Manifold Approximation and Projection (UMAP). Using UMAP, all cells underwent dimensional reduction and were clustered in a two-dimensional space based on shared features. Firstly, the Azimuth algorithm was used to map the data to the reference cell set of PBMC, and then combined with specific high expression genes to manually determine the cell type. Specifically, classic biomarkers for specific cell types were used to identify the cells in different clusters. The FindAllMarkers function in Seurat was used to identify the 50 most highly expressed genes in each cluster of cells, providing a comprehensive understanding of cell types based on the top gene and literature. When clustering for the first or the second time, clusters expressing two or more classic markers and marginalized cells were considered doublets and excluded from subsequent analysis. Use Milo algorithm62 to divide the cells of the control group and BA.2-BTI-6m group into different neighborhoods and calculate their spatial distribution differences, map** them to different cell types. The key parameters for executing the Milo algorithm are k = 10 and d = 30. In addition, the proportion of cell types for each sample was calculated based on the conventional cell percentage and their differences between groups were calculated using the rank sum test. The Findmarkers() function in the Seurat package was used to identify differentially expressed gene (DEG)s between distinct cell groups, using a standard of |logFC|> 0.25 and FDR < 0.01. DEGs only contain genes expressed in at least 25% of cells of the control group or infection group. The ClusterProfiler R package facilitated Gene Ontology and KEGG enrichment analyses and visualization of DEGs. The AddModuleScore() function of Seurat was used to calculate the activity scores of different gene sets in single cells. The gene set was sourced from the msigdb R package (Antigen processing and presentation (hsa04520), JAK_STAT_signaling (hsa04630), B cell activation (GO:0042113), B cell receptor signaling (GO:0050853), positive regulation of Treg activity (GO:0045591), response interferon (GO:0034341), protein processing (GO:0016485) and coagulation regulation (GO:0007597, GO:0050819, GO:0050820, GO:0050818). T cell toxicity activity was defined by the following gene sets: PRF1, IFNG, GNLY, NKG7, GZMB, GZMA, GZMH, KLRK1, KLRB1, KLRD1, CTSW, and CST7. The tissue specific gene set based on proteomics comes from the research of Gutmann et al.23 and Li et al.24. Using human GRCh38 as the reference genome, the Cell Ranger vdj pipeline was used to identify the TCR/BCR clonotype and quantify VDJ gene expression. For TCR, we only retained cells with at least one productive TCRα chain (TRA) or TCRβ chain (TRB) for subsequent analysis. Where a cell had two or more paired TRA or TRB chains, we only retained the one with the highest basal expression. Clonotypes were defined based on their unique CDR3 amino acid sequence, and each unique TRA/TRB/TRA-TRB pair was defined as a clonotype. For BCR analysis, we retained only cells with at least one productive heavy chain (IGH) and IGK/IGL for subsequent analysis. When a cell had two or more paired IGH or IGK/IGL chains, only those with the highest basal expression were retained. Each unique pair IGH-IGK/IGL was defined as a clonotype. The scRepertoire R package (https://github.com/ncborcherding/scRepertoire) was used to analyze the single-cell immune repertoire and calculate the clonal diversity of the samples based on the aroma index. Based on the cell barcode information, clonotypes with TCR or BCR were mapped onto the cell UMAP map. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.Cell type annotation
Cell difference abundance analysis
Differential gene identification and functional analysis
Gene set activity score of individual cells
TCR/BCR analysis
Reporting summary
Data availability
The single-cell sequencing data generated in this study have been deposited in the Genome Sequence Archive63 database under accession code HRA004484 (https://ngdc.cncb.ac.cn/gsa-human). The raw single-cell sequencing data are protected and restrictedly available due to data privacy laws. The processed single-cell sequencing data are available at the Gene Expression Omnibus database (access number: GSE240694). The mass spectrometry proteomics data generated in this study have been deposited to the ProteomeXchange Consortium via the iProX partner repository under the accession code PXD044441 (http://proteomecentral.proteomexchange.org). The manuscript did not generate original code and the analysis process link can be found in the manuscript methods or contacted by the authors. Source data are provided with this paper.
References
Timeline: WHO’s COVID-19 response. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline (2022).
Kandeel, M., Mohamed, M. E. M., Abd El-Lateef, H. M., Venugopala, K. N. & El-Beltagi, H. S. Omicron variant genome evolution and phylogenetics. J. Med. Virol. 94, 1627–1632 (2022).
Fan, Y. et al. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct. Target Ther. 7, 141 (2022).
Kumar, S., Thambiraja, T. S., Karuppanan, K. & Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 94, 1641–1649 (2022).
COVID- 19 Clinical and Surveillance Data--Dec 9, 2022 to Jan 23, 2023, China. https://en.chinacdc.cn/news/latest/202301/t20230126_263523.html (2023).
GISAID - NextStrain. https://gisaid.org/phylodynamics/global/nextstrain/ (2023).
Crook, H., Raza, S., Nowell, J., Young, M. & Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 374, n1648 (2021).
CDC. Long COVID or Post-COVID Conditions. Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (2023).
Thaweethai, T. et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 329, 1934–1946 (2023).
Abdullah, F. et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 116, 38–42 (2022).
Wolter, N. et al. Clinical severity of SARS-CoV-2 Omicron BA.4 and BA.5 lineages compared to BA.1 and Delta in South Africa. Nat. Commun. 13, 5860 (2022).
Wolter, N. et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet 399, 437–446 (2022).
Cai, J. et al. A one-year follow-up study of systematic impact of long COVID symptoms among patients post SARS-CoV-2 omicron variants infection in Shanghai, China. Emerg. Microbes Infect. 12, 2220578 (2023).
Muik, A. et al. Omicron BA.2 breakthrough infection enhances cross-neutralization of BA.2.12.1 and BA.4/BA.5. Sci. Immunol. 7, eade2283 (2022).
Al-Aly, Z., Bowe, B. & **e, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28, 1461–1467 (2022).
Morgan, P. China’s COVID-19 Vaccine Supplies to the Global South: Between Politics and Business (Policy Press, 2022).
Government orders 4.3 million further doses of COVID-19 antivirals. Pharm. J. https://doi.org/10.1211/pj.2021.1.121425 (2021).
Zhu, F.-C. et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 395, 1845–1854 (2020).
Li, J.-X. et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir. Med 10, 739–748 (2022).
Chen, J. et al. Inflammatory stress in SARS-COV-2 associated Acute Kidney Injury. Int. J. Biol. Sci. 17, 1497–1506 (2021).
Kratofil, R. M., Kubes, P. & Deniset, J. F. Monocyte Conversion During Inflammation and Injury. Arterioscler. Thromb. Vasc. Biol. 37, 35–42 (2017).
Mitchell, A. J., Roediger, B. & Weninger, W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell. Immunol. 291, 22–31 (2014).
Gutmann, C. et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat. Commun. 12, 3406 (2021).
Li, Y. et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J. Clin. Invest. 131, e148635 (2021).
Reyes, M. et al. An immune-cell signature of bacterial sepsis. Nat. Med. 26, 333–340 (2020).
Reyes, M. et al. Plasma from patients with bacterial sepsis or severe COVID-19 induces suppressive myeloid cell production from hematopoietic progenitors in vitro. Sci. Transl. Med. 13, eabe9599 (2021).
Li, J. et al. Circulating prolactin concentrations and risk of type 2 diabetes in US women. Diabetologia 61, 2549–2560 (2018).
Borba, V. V., Zandman-Goddard, G. & Shoenfeld, Y. Prolactin and Autoimmunity. Front. Immunol. 9, 73 (2018).
Dong, T., Zhi, L., Bhayana, B. & Wu, M. X. Cortisol-induced immune suppression by a blockade of lymphocyte egress in traumatic brain injury. J. Neuroinflammation 13, 197 (2016).
Das, C., Rout, M. K., Wildering, W. C. & Vijayan, M. M. Cortisol modulates calcium release-activated calcium channel gating in fish hepatocytes. Sci. Rep. 11, 9621 (2021).
Widmer, I. E. et al. Cortisol Response in Relation to the Severity of Stress and Illness. J. Clin. Endocrinol. Metab. 90, 4579–4586 (2005).
Kawohl, W. & Nordt, C. COVID-19, unemployment, and suicide. Lancet Psychiatry 7, 389–390 (2020).
**ong, J. et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 277, 55–64 (2020).
Klein, J. et al. Distinguishing features of Long COVID identified through immune profiling. medRxiv, https://doi.org/10.1101/2022.08.09.22278592 (2022).
Chandel, A. et al. Association of D-dimer and Fibrinogen With Hypercoagulability in COVID-19 Requiring Extracorporeal Membrane Oxygenation. J. Intensive Care Med. 36, 689–695 (2021).
Mazzeffi, M. A., Chow, J. H. & Tanaka, K. COVID-19 Associated Hypercoagulability: Manifestations, Mechanisms, and Management. Shock 55, 465–471 (2021).
Kichloo, A. et al. COVID-19 and Hypercoagulability: A Review. Clin. Appl. Thromb. Hemost. 26, 1076029620962853 (2020).
Levi, M. & Ten Cate, H. Disseminated intravascular coagulation. N. Engl. J. Med. 341, 586–592 (1999).
Lechner-Scott, J., Levy, M., Hawkes, C., Yeh, A. & Giovannoni, G. Long COVID or post COVID-19 syndrome. Mult. Scler. Relat. Disord. 55, 103268 (2021).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Astin, R. et al. Long COVID: mechanisms, risk factors and recovery. Exp. Physiol. 108, 12–27 (2023).
Tian, W. et al. Immune suppression in the early stage of COVID-19 disease. Nat. Commun. 11, 5859 (2020).
Mehandru, S. & Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 23, 194–202 (2022).
Al-Aly, Z., **e, Y. & Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259–264 (2021).
Suryawanshi, P. et al. Lymphopenia with Altered T Cell Subsets in Hospitalized COVID-19 Patients in Pune, India. Viral Immunol. 36, 163–175 (2023).
Asakura, H. & Ogawa, H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 113, 45–57 (2021).
Wang, X. et al. Neutralization of Omicron BA.4/BA.5 and BA.2.75 by booster vaccination or BA.2 breakthrough infection sera. Cell Discov. 8, 110 (2022).
Quandt, J. et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci. Immunol. 7, eabq2427 (2022).
Evans, J. P. et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 14, eabn8057 (2022).
Hachmann, N. P. et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 387, 86–88 (2022).
Qu, P. et al. Neutralization of the SARS-CoV-2 Omicron BA.4/5 and BA.2.12.1 Subvariants. N. Engl. J. Med. 386, 2526–2528 (2022).
Qu, P. et al. Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe 31, 9–17.e3 (2023).
Cao, Y. et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 182, 73–84.e16 (2020).
Ren, X. et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell 184, 1895–1913.e19 (2021).
Delorey, T. M. et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 595, 107–113 (2021).
Wei, J. & Hui, A. Review of Ribosome Interactions with SARS-CoV-2 and COVID-19 mRNA Vaccine. Life 12, 57 (2022).
Yuan, J. et al. 3’UTR of SARS-CoV-2 spike gene hijack host miR-296 or miR-520h to disturb cell proliferation and cytokine signaling. Front. Immunol. 13, 924667 (2022).
Zhao, X. et al. Omicron SARS-CoV-2 Neutralization from Inactivated and ZF2001 Vaccines. N. Engl. J. Med. 387, 277–280 (2022).
Zhao, X. et al. Effects of a Prolonged Booster Interval on Neutralization of Omicron Variant. N. Engl. J. Med. 386, 894–896 (2022).
Chen, Z. et al. Synergistic effects of mechanical stimulation and crimped topography to stimulate natural collagen development for tendon engineering. Acta Biomater. 145, 297–315 (2022).
Feng, Q. et al. The anti-aging effects of Renshen Guben on thyrotoxicosis mice: Improving immunosenescence, hypoproteinemia, lipotoxicity, and intestinal flora. Front. Immunol. 13, 983501 (2022).
Dann, E., Henderson, N. C., Teichmann, S. A., Morgan, M. D. & Marioni, J. C. Differential abundance testing on single-cell data using k-nearest neighbor graphs. Nat. Biotechnol. 40, 245–253 (2022).
Chen, T. et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genomics Proteom. Bioinforma. 19, 578–583 (2021).
Acknowledgements
This study was supported by the National Key R&D Program of China (2020YFA0907102, G.F.G.), the National Natural Science Foundation of China (82170004, Y.M. and 82222040, X.Z.), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020092, X.Z.), CAS Project for Young Scientists in Basic Research (YSBR-083, X.Z.), the China Postdoctoral Science Foundation (No. 2023M743729, Yanhua L. and 2022M723344, S.Qin) and the Air Force Special Medical Center Science and Technology Booster Program (2022ZTYB39, L.D.). The authors thank all the participants recruited for their contributions to this study. We sincerely thank Dr. Daniel Montiel-Garcia (Ph.D.) from Scripps Research and Mohammed H. Althunayan (MPH) from Michigan University for their time and kind help in language editing and smoothing the manuscript. The authors sincerely thank OE Biotech Co., Ltd., (Shanghai, China) for providing single-cell RNA-seq and TCR/BCR sequencing analysis.
Author information
Authors and Affiliations
Contributions
G.F.G., Y.M., X.Z., Yanhua L. and S.Qin conceptualized the study. Yanhua L. and S.Qin wrote the original draft. G.F.G., Y.M. and X.Z. supervised the study. Yanhua L. and S.Qin did data analysis and figure making with the assistance of S.Qiao. L.D., S.Quan, Ying L., F.F. and Yanhua L. were responsible for sample collection and clinical data assembly and assessments on pathological features. Yanhua L. and D.Y. interpreted the pathological characteristics. Yanhua L., S.Qiao, X.W., P.G. and Y.H. did pseudovirus neutralization assays. G.F.G., Y.M., X.Z., Yanhua L., S.Qin and S.Qiao curated the data. G.F.G., Y.M., X.Z., Yanhua L. and L.D. are responsible for funding resources. All authors reviewed and edited the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Amanda Oliver and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Qin, S., Dong, L. et al. Long-term effects of Omicron BA.2 breakthrough infection on immunity-metabolism balance: a 6-month prospective study. Nat Commun 15, 2444 (2024). https://doi.org/10.1038/s41467-024-46692-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46692-z
- Springer Nature Limited