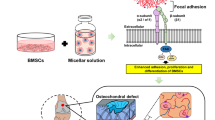
Abstract
Injectable biomaterials have garnered increasing attention for their potential and beneficial applications in minimally invasive surgical procedures and tissue regeneration. Extracellular matrix (ECM) hydrogels and porous synthetic polymer microspheres can be prepared for injectable administration to achieve in situ tissue regeneration. However, the rapid degradation of ECM hydrogels and the poor injectability and biological inertness of most polymeric microspheres limit their pro-regenerative capabilities. Here, we develop a biomaterial system consisting of elastic porous poly(l-lactide-co-ε-caprolactone) (PLCL) microspheres mixed with ECM hydrogels as injectable composites with interleukin-4 (IL-4) and insulin-like growth factor-1 (IGF-1) dual-release functionality. The developed multifunctional composites have favorable injectability and biocompatibility, and regulate the behavior of macrophages and myogenic cells following injection into muscle tissue. The elicited promotive effects on tissue regeneration are evidenced by enhanced neomusle formation, vascularization, and neuralization at 2-months post-implantation in a male rat model of volumetric muscle loss. Our developed system provides a promising strategy for engineering bioactive injectable composites that demonstrates desirable properties for clinical use and holds translational potential for application as a minimally invasive and pro-regenerative implant material in multiple types of surgical procedures.
Similar content being viewed by others
Introduction
Trauma and tumor resection result in tissue defects that require morphological and functional restoration. To promote in situ tissue regeneration, biomaterials have been widely employed as strategies to modulate host cell behavior and stimulate endogenous regeneration potential1,2. Biomaterial application or implantation is a simplistic, effective, and clinically translational approach that avoids limitations associated with traditional tissue engineering1,3. Various implantable biomaterials have been developed to improve tissue regeneration, but most of require open surgery that leads to risk of postoperative infection and poor healing outcomes. In contrast, injectable hydrogels and microspheres provide minimally invasive means of achieving tissue regeneration4,5 coupled with the advantages of minor tissue injury, fast recovery, convenience of operation, and clinical safety. However, when injected, microspheres or hydrogels suffer from unavoidable drawbacks, such as deformation and rapid degradation, respectively5,6. Therefore, in recent years there has been considerable efforts made to develop functional injectable biomaterials that promote in situ tissue regeneration7,8.
Extracellular matrix (ECM) hydrogels and synthetic polymer microspheres are commonly used as injectable biomaterials for the repair of injured tissues4,55. In addition, ECM was also able to stimulate endogenous NK cells to produce chemokine Xcl-1 and recruit DC cells to promote the secretion of cytokines such as IL-4, IL-10, and IL-33 in defect sites, thereby improving the myofibers regeneration56. However, the capability of ECM scaffolds to recruit and support resident myogenic stem cell ingrowth and to promote tissue regeneration is still limited. The second aspect was the controlled release of IGF-1 sustained the activation of myosatellite cell proliferation and differentiation, myofiber survival (Fig. 5, Supplementary Fig. 12), and hypertrophy-related signaling pathways38,57,58,59,60, such as the PI3K/Akt57,59,60,61, IGF-158 and mTOR61 pathways, thus promoting myofiber formation. In addition, numerous studies have shown that pro-repair macrophage paracrine factors can also encourage endothelialization62, vascularization63, and neural regeneration64, which are consistent features also demonstrated in our study.
The injectability and in situ pro-regenerative potential of our functionalized composite system was validated in a small animal model. However, further validation is still needed in larger models and under pathogenic conditions of disease models. The effect of elastic porous microspheres combined with other select tissue-specific ECM for the repair of distinct tissue types after injury or surgical traumas, especially tissues under mechanical load such as articular cartilage and intervertebral discs, is a natural future direction to explore the scope of our developed composite system. Cell-laden porous microspheres strategies were used to enhance tissue regeneration11,65, but it was noted that shear stress during injection had consequential outcomes on microsphere integrity and unavoidable cell apoptosis or loss. Therefore, pre-seeding of elastic porous PLCL microspheres with tissue-specific or stem/progenitor cells in combination with the support of an ECM hydrogel carrier may offer an alternative method for improving cell survivability post-implantation. Also, the introduction of hydroxyl, amino, polypeptide (RGD) on the surface of the elastic microspheres is expected to improve the efficiency of cell adhesion and drug delivery, which are worthy of further study. In this work, we constructed functionalized injectable composites, which employed the complementary advantages of two different material structures and the synergistic effect of dual bio-factors to achieve endogenous tissue regeneration. We demonstrated the injectability, biocompatibility, and in situ pro-regenerative potential of the composites in vitro and in vivo. Furthermore, the regenerative potential of composites for other tissue defects, including nucleus pulposus, cartilage etc., through minimally invasive therapeutic manner is worthy of investigation in the future. Our study also opens a new avenue for designing and fabricating novel injectable bioactive materials.
Methods
Ethical regulations statement
All animal experiments were approved by the animal experiments ethical committee of Nankai University, Tian**, China (2022-SYDWLL-000432) and met the requirements of the Guidelines for Care and Use of Laboratory Animals.
Animal experiments
Seventy-five male Sprague Dawley rats (aged 8–10 weeks with the weight range of 280–300 g), 30 male Sprague Dawley rats (aged 4–5 weeks with the weight range of 120–150 g) and 42 male Sprague Dawley mammary rats (5 days) were used in this study. All animals were purchased from SPF Biotechnology Co., Ltd. (Bei**g, China). Rats were randomly divided into cages (3 rats per cage) and adapted to a pellet-based diet with tap water for 7 days in an SPF environment. The ambient temperature was maintained at 23 ± 3 °C, humidity at 40–70%, and cycle light/dark for 12 h. Rats were fed a standard food diet and tap water. And during the feeding period, the animal room was cleaned regularly. Rats were fasting 12 h pre-surgery but can have water. After surgery, all experimental animals were lying sideways on a heating blanket (37 °C) to maintain their smooth breathing and body temperature within 2 h. Paid attention to maintaining a quiet environment until the animals were completely awake. All experimental animals were fasting 12 h post-surgery but can drink freely. During the wound recovery period, we also paid attention to animals’ physical conditions, such as feeding, excretion, surgical infection, and suture status. In addition, we recorded the local reaction of the injection site after injecting different materials in the rat subcutaneous injection model and VML model, including visible swelling, redness, edema, and abnormal color. Sixty-six rats were used to construct VML models. Nine rats were used to evaluate the cell infiltration and immunomodulatory properties of composites. 42 male Sprague Dawley rats (120–150 g) were used to isolate primary macrophages. All mammary rats were used to isolate primary muscle satellite cells. For all experiments n = 3 rats per group was set for obtaining numerical data.
Materials
Poly (l-lactide-co-ε-caprolactone) (PLCL) (50:50) with viscosity of 2.6–2.8 and poly (lactic acid/glycolic acid) (PLGA) (50:50) with molecular weight of 50 kDa were purchased from Daigang Biomaterial Co., Ltd (**an, China), poly(caprolactone) (PCL) with molecular weight of 80 kDa and gelatin (G9391) were purchased from Sigma (USA). Span-80 was obtained from JSENB International Trade Co., Ltd (Hong Kong, China). Polyvinyl alcohol (PVA) was purchased from Dehang Wuzhou Technology Co., Ltd (Bei**g, China). Dopamine hydrochloride (98%) was obtained from Innochem Technology Co., Ltd (Bei**g, China). Cardiotoxin (CTX) was purchased from Guchen Biotechnology Co., Ltd (Shanghai, China). Rat-derived IGF-1 and Interleukin-4 (IL-4) were purchased from Biogot Technology, co, Ltd (Nan**g, China). 24-well cell climbing films, sodium dodecyl sulfate (SDS), ribonuclease (RNase), deoxyribonuclease I (DNase I), α-Galactosidase (α-GAL) kit, type I collagenase (C8140), sodium citrate antigen retrieval solution (50×) and 4% paraformaldehyde solution were obtained from Solarbio (Bei**g, China). DNA quantitative kit was purchased from Yeasen Biotechnology Co., Ltd (Shanghai, China). Rat IL-4 and IGF-1 Elisa kits were purchased from Aibotech Biotechnology co, Ltd (Wuhan, China). DAPI fluoromount-G was purchased from Southern Biotech (USA). Alcohol, dichloromethane, and xylene were purchased from Tian** Chemical Reagent Company (Tian**, China). 3-0 and 9-0 nylon needle sutures were purchased from Ningbo Medical Needle Co., Ltd (Zhejiang, China). 3 M Tegaderm™ films were purchased from Polite Trading Co., Ltd (Tian**, China). Paraffin wax was purchased from Leica Biosystems Richmond (USA). Flou-8 AM fluorescence probe (C0012) was obtained from Applygen Gene Technology Co., Ltd (Bei**g, China). The information on primary and secondary antibodies is exhibited in Supplementary Table 1.
Preparation of porous polymeric microspheres and dopamine modification
Different porous polymer microspheres were prepared using a microfluidic device. The water-in-oil (W-O) emulsion was prepared through emulsifying 2 mL aqueous gelatin (2.5–10.0%) and 100 µL span-80 in a 7.5 mL different polymer solution (2%) using a cell ultrasonic-homogenizer (JY92-IIN, China) at 30% ultrasonic power for 1 min. Subsequently, the prepared emulsion was introduced as a discontinuous phase into the fluidic device, and aqueous PVA solution (1%) served as the continuous phase. Then, the water-in-oil-in-water (W-O-W) droplets were formed at the tip of the needle, and the polymer-gelatin microspheres were obtained in ice water and followed by stirring at 75 r/min for 12 h to evaporate excess dichloromethane solvent, and the gelatin component was removed in water at 40 °C. Porous polymer microspheres with different diameters and pore sizes were obtained by adjusting the needle size (18G, 20G, 22G, 24G, 26G) and gelatin concentration (2.5%, 5.0%, 7.5%, and 10.0%).
Porous PLCL microspheres were added in 0.5 mg/mL dopamine tris-buffer (pH = 8.5), 75 r/min shaker for 12 h. After modification, the tris-buffer removed, and the microspheres were cleaned with sterile water three times.
Characterization of the porous polymeric microspheres
Stereoscopic microscopy and scanning electron microscopy (SEM) were used to observe the macro- and microscopic structures of PM. Atomic force microscopy (AFM) was used to observe the roughness of porous PLCL microspheres before and after dopamine modification, and XPS spectroscopy was used to observe the N 1s peak at 398.4 eV. The pore areas were calculated in SEM images of PM using image por-plus, and the relative pore sizes were converted by the following formula.
The porosity of the PM was measured by liquid displacement method. PM was immersed in the dehydrated ethanol (initial volume V0) for 5 min. V1 was defined as the total volume of the system when the microspheres were immersed in the dehydrated alcohol. Then, microspheres were removed and the volume of the residual ethanol was recorded as V2. The porosity of PM was defined as the following equations:
Polymer microspheres were loaded in a 2.5 mL syringe with a 21G needle and injected rapidly through the glass capillary tube with an inner diameter of 0.8–1.0 mm. The morphology images of different microspheres were captured through an inverted microscope after injection. The elasticity of different polymeric microspheres (PCL, PLGA, and PLCL) was tested using tweezers by performing a single press for 30 s and 2 min, and multiple press for 15 times. The morphological photographs were recorded through a stereoscopic microscope, and the results were analyzed using Image-Pro Plus.
mECM hydrogel preparation
Adult porcine skeletal muscle was purchased from Tian** Er-shang Yingbin Meat Food Co. Porcine skeletal muscle was cut into small pieces and eluted with 1% SDS for 72 h, and then washed in sterile water for 48 h. DNase and RNase mixture were used to remove DNA and RNA at constant shaking speed of 150 r/min for 6 h at 37 °C. After washing for 48 h, the decellularized samples were freeze-dried and ground into powder using a cryogenic tissue grinder (Shanghai **gxin Industrial Development Co., Ltd, China). The decellularization process can also refer to our previous study29,66. Then, mECM powder was dissolved in 0.01 M HCl and digested with pepsin at a ratio of 10:1 for 48 h. After digestion, the pH was adjusted to 7.4. The mECM pre-gels were mixed with 10 × PBS solution of 10% of its total volume and incubated at 37 °C to form hydrogels.
mECM characterization
Quantitative analysis of the remaining DNA content of samples was performed using a Quant-iT PicoGreen Assay (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions. Specifically, DNA was extracted from native muscle and decellularized muscle ECM prior to quantification. After diluting the DNA of samples and fluorescent dye, 125 μL of gradient DNA standard solution and the test solution were added to the 96-well plate, then 125 μL of the diluted DNA fluorescent dye and reacted for 5 min. A multifunctional microplate reader (excitation wavelength: 480 nm, emission wavelength: 520 nm) was used to measure the fluorescence intensity of samples and quantified by a standard curve of DNA. Furthermore, the remaining α-galactosidase (α-Gal) samples were performed using α-GAL activity detection kit (Solarbio) according to the manufacturer’s instructions. H&E and Masson’s histological staining was performed to observe the morphology of myofibers and collagen composition. DAPI fluorescence staining was used to detect cells before and after decellularization.
Preparation and characterization of the mECM + PM composites
mECM and porous PLCL microspheres were physically mixed at different mass ratios (30:1, 10:1, 5:1, 3:1) to obtain the mECM + PM composites. Then, different mECM+PM composites were loaded into 2.5 mL syringes, the sedimentation of microspheres was recorded at different time points (0, 5 min, 30 min, 6 h, 24 h and 48 h). SEM was used to detect the distribution of PM inside the mECM hydrogel in the lyophilized state. Under wet-state conditions, porous PLCL microspheres were labeled with rhodamine, mECM hydrogels were labeled with FITC, and three-dimensional spatial structures of composites were observed by a confocal laser microscope (Leica, SP8, Germany). Rheological measurements of the pure mECM hydrogel and mECM + PM composites (different mass ratios or different porosity of microspheres) were carried out using a rheometer at different temperatures from 10 to 40 °C. The storage modulus (G’) and loss modulus (G”) were measured using flat plates (10 mm), the shear strain was set to 1%, and the shear frequency was set to 12 rad/s.
Fabrication of the mECM@IL4 + PM@IGF1 composites and release profile
1.5 mg dopamine-modified porous PLCL microspheres were placed in 500 μL IGF-1 solution (500 ng/mL) under alkaline conditions, incubated for 24 h on a shaker at 4 °C, and then freeze-dried to obtain PM@IGF-1 microspheres, 4 μL of 25 μg/ml IL-4 (total 100 ng) was directly added into the 500 μL mECM pre-gel. Finally, 500 μL pre-gel was mixed with 1.5 mg microspheres to obtain the mECM@IL4 + PM@IGF1 composites. For the IL-4 and IGF-1 release assay, 500 μL functionalized composites were placed in 1 mL PBS solution and incubated on a shaker at 37 °C. At different time points (3 h, 12 h, 1 day, 2 days, 3 days, 5 days, 7 days, 10 days, 14 days, 20 days), 500 μL supernatant was collected and added with 500 μL PBS. The collected samples were stored at −80 °C. Finally, the release of IL-4 and IGF-1 was measured using ELISA kits. In brief, 100 μL sample diluent was added to the antibody-coated ELISA plate and incubated for 2 h. After cleaning with wash buffer, 100 μL biotin conjugate antibody was added and incubated for 1 h. Subsequently, 100 μL streptavidin-HRP solution was added and incubated for 30 min. After cleaning the ELISA plate, 100 μL TMB substrate was added and incubated for 20 min. Finally, 50 μL stop solution was directly added to stop reaction, and the absorbance value was measured at 450 nm.
IGF-1 release behavior after injection and deformation
3 mg PM@IGF-1 microspheres were resuspended in 1 mL PBS solution for injection test. Then, the supernatants in injection group and non-injection group were collected to detect the release of IGF-1 after 24 h.
3 mg PM@IGF-1 microspheres were resuspended in 1 mL PBS and placed on a 12-well plate for parallel plate compression test. The supernatants were collected firstly at different time points (0, 12 h, 24 h, 72 h, 120 h, 168 h and 240 h). Then, a 20 g weight placed on a glass plate was used to compress the microspheres for 30 s. After collecting 500 μL supernatant, the fresh PBS was supplemented to 1 mL, and cultivated at 37 °C. Finally, ELISA kit was used to detect the IGF-1 release.
Primary cell isolation and culture
Primary bone marrow-derived macrophages (BMDMs): male SD rats (120–150 g) were sacrificed, the femur and tibia were cut, and all cells were flushed out with basic medium. Then, cell suspension solution was passed through 70 μm and 40 μm microporous filter membranes. After centrifugation at 160 × g for 5 min, the supernatant was removed, and 10 mL erythrocyte lysate was added for 10 min and shake every 5 min, and then collected cells were seeded on 75 mL culture flask with 15% inactivated fetal bovine serum after centrifugation at 160 × g for 5 min. Subsequently, suspended cells were collected after 3–4 days of culture. The suspended cells were centrifuged, collected and inoculated in a 75 mL flask and cultured by adding 20 ng/mL GM-CSF macrophage-stimulating factor. The culture medium was changed every 3 days, and BMDMs were obtained after 7 days.
Primary muscle satellite cell extraction and culture: male SD mammary rats (5 days) were sacrificed, and all leg muscles were cut into small pieces, then treated with 0.2% type I collagenase for 40 min, and centrifuged at 500 × g for 1 min. The supernatant was removed, incubated with trypsin containing 0.2% EDTA at 37 °C for 40 min, and then terminated digestion by adding complete medium. The remaining impurities were removed by passing through 70 μm and 40 μm filters. The cells were then centrifuged at 160 × g for 5 min and resuspended with 3 mL medium. 2 mL of 80% percoll isolate, 8 mL of 20% percoll isolate, and 3 mL cell resuspension were slowly added into the centrifuge tube in sequence, and centrifuged at 3400 × g for 6 min. Cells from the middle layer of 20% and 80% of the isolate were collected for inoculation, the suspended cells were reinoculated using the differential adhesion method for 1 h and 2 h, and the suspended cells were taken and reinoculated. Cells at 70% confluence were passaged to prevent the differentiation of muscle satellite cells.
The regulation of macrophage polarization in vitro
The composites were cocultured with BMDMs using Transwells, the extracted primary macrophages were inoculated at 5 × 104 cells/well in the lower chamber of 24-well plates. Different composites (200 μL) containing 0.6 mg microspheres with additional 200 μL 1640 basal medium were added to the upper chamber of the transwells, namely mECM + PM, mECM@IL4 + PM, mECM + PM@IGF1 and mECM@IL4 + PM@IGF1, and 100 ng IL-4 was mixed in each IL-4 loaded composites. 200 μL 1640 basal medium was added into the upper chamber as negative group, and 200 μL 1640 basal medium containing 100 ng IL-4 was set as positive group. After coculture for 1 and 3 days, CD68 and CD206 co-staining was performed, the images were captured under a fluorescence microscope (Zeiss Axio Imager Z1, Germany), and the statistical analysis was performed using Image-Pro Plus and GraphPad 6.0.
Flow cytometric analysis: The extracted BMDMs were inoculated in six-well plates at 1 × 105 cells/well, and different materials were added to the upper chamber of the Transwell. 2 mL 1640 basal medium mixed with different composites (500 μL) containing 1.5 mg microspheres were added to the upper chamber of the transwells, namely mECM + PM, mECM@IL4 + PM, mECM + PM@IGF1 and mECM@IL4 + PM@IGF1, 100 ng IL-4 was loaded in each IL-4 delivery composites. 2 mL 1640 basal medium was added into the upper chamber as negative group, and 2 mL 1640 basal medium containing 100 ng IL-4 was set as positive group. After 1 and 3 days, the primary macrophages in the lower chamber were collected using a cell scraper and centrifuged at 160 × g for 5 min. The collected cells were incubated with Alexa Fluor® 647 CD206 antibody (1:200, 141712, Biolegend) and FITC® 488 CD68 antibody (1:100, 137012, Biolegend) for 30 min. Afterwards, the cells were washed twice with staining buffer, followed by resuspension in 800 μL staining buffer. The stained cells were detected using a FACSCalibur flow cytometer (BD Biosciences) and CellQuest software (Pharminogen). Data were analyzed using FlowJo software.
Cytokine measurement: BMDMs at a density of 2.5 × 105 cells/well were seeded on the lower chamber of six-well plate transwells. BMDMs were first educated and cultured with 1640 medium containing 5% heat-inactivated FBS. Other cultivation conditions were consistent with flow cytometry analysis. After incubation for 1 and 3 days, the cell supernatants were collected and centrifuged at 250 × g for 10 min, and stored at −80 °C. The samples were detected using magnetic bead (Luminex) multiplexed cytokine assays by Youning Weisheng Technology Co., Ltd (Shanghai, China).
The synergistic effects of IL-4 and IGF-1 on the proliferation of myogenic cells
L6 cells (Rat, ATCC, CRL-1458) were seeded on 96-well plates at a density of 2 × 103 cells/well. After culturing for 12 h, the supernatant was removed, and 100 µL DMEM complete medium containing single factors (IL-4 or IGF-1) or dual factors (IL-4 + IGF-1) with different concertation were added and cultured for 1 and 3 days. At different time points, CCK-8 dilution in DMEM (1:10) was added to 96-plate for 40 min. The optical density (OD) value at 450 nm was detected with a microplate reader (Bio-Rad, USA) to evaluate cell proliferation.
Interaction experiments of composites, BMDMs, and L6/muscle satellite cells
Cell viability assay: Different composites including PM@IGF1, mECM + PM, mECM + PM@IGF1 and mECM@IL4 + PM@IGF1 were added to 3 mL of DMEM medium and soaked for 1 day, and upper conditioned medium were centrifuged and collected. L6 cells were seeded on 96-well plates at a density of 2 × 103 cells/well. After culturing for 12 h, the supernatant was removed, and 100 µl of different conditioned medium containing 10% FBS were added and cultured for 1, 3, 5, and 7 days. 100 µl DMEM complete medium was added as negative control, and 100 ng/mL IGF-1 DMEM complete medium was set as positive control. At different time points, CCK-8 dilution in DMEM (1:10) was added to 96-plate for 40 min. The optical density (OD) value at 450 nm was detected with a microplate reader (Bio-Rad, USA) to evaluate cell viability.
Live/dead staining: The composites were cocultured with L6 cells using Transwells. L6 cells were inoculated at 1.0 × 104 cells/well on cell climbing films in the lower chamber of 24 wells. Different materials were added in the upper chamber of the transwells, including 200 μL DMEM basal medium, 200 μL mECM + PM composites, 200 μL DMEM basal medium mixed with 0.6 mg PM@IGF1, 200 μL mECM + PM@IGF1 composites, 200 μL mECM@IL4 + PM@IGF1 composites, and 200 μL DMEM basal medium containing 100 ng/mL IGF-1 as positive control. Dead/live staining was used to observe L6 cell survival after culturing for 3, 5, and 7 days, the images were captured under a fluorescence microscope (Zeiss Axio Imager Z1, Germany), the number of dead cells was calculated using Image-Pro Plus.
Apoptosis assay: L6 cells were inoculated at 1.0 × 104 cells/well in the lower chamber of 24-well plates under 2% low-serum culture, other cultivation conditions were consistent with live/dead staining. After 7 days of coculture, the cells were washed with PBS and fixed with 4% paraformaldehyde solution. Then, L6 cell apoptosis was detected using a one-step TUNEL Cy5 apoptosis detection kit (k1135, APE×BIO).
Primary muscle satellite cells injury model: To determine the appropriate injury conditions, satellite cells were treated with different CTX concentrations (0, 0.3 µM, 0.6 µM and 1.0 µM) for 2.0 ± 0.5 h or 4.0 ± 0.5 h. The CTX solutions were removed after treatment, and cells were treated with fluo-8am Ca+ fluorescence probe for 30 min according to the manufacturer’s instructions. Then, the intracellular Ca2+ fluorescence intensity was detected at 490 nm using a multifunctional enzyme marker. In addition, Ca2+ fluorescence images were captured using a fluorescence microscope (Zeiss Axio Imager Z1, Germany). 0.3 μM CTX concentration was selected for subsequent experiment. Finally, we used the 24-well plate transwell to evaluate the effect of BMDMs cooperated with composites on the differentiation behavior of injured muscle satellite cells. Satellite cells were inoculated in the lower chamber at 2.5 × 104 cells/well for 3 days in advance. After 0.3 μM CTX treatment, BMDMs (2 × 104 cells/well) were directly added in the lower chamber of the transwells and co-cultured with satellite cells, and composites were directly placed in the upper chamber which were named as BMDMs, composites and BMDMs/composites, respectively. Cells with and without CTX treatment were set as control group. Subsequently, DMEM medium containing 10% FBS and 2% horse serum added in the lower chamber was applied for cell differentiation. After 7 days of coculture, phalloidin (1:80, Solarbio, ca1620), desmin (1:200, Santa, sc23879) and CD206 (1:300, Abcam, ab64693) fluorescence co-staining was performed to observe the ability of primary muscle satellite cell differentiation, the fluorescence images were captured using a total internal reflection fluorescent microscope TIRF & Thunder. The myotube length and area, the nuclei number per myotube and the number of CD206+ cells were analyzed using Image-Pro Plus.
Hemolysis detection ex vivo
Fresh rat blood was collected and anticoagulated using 1% heparin sodium. 0.2 mL blood was added to the centrifuge tube containing 10 mL normal saline and different samples including mECM+PM composites (200 µL) and mECM@IL4 + PM@IGF1 composites (200 µL, 500 µL). 10 mL normal saline were used as the negative control group, and 10 mL deionized water was used as the positive control group. After incubating for 30 min, 4 mL of suspension in each group was collected and centrifuged at 1040 × g for 5 min. The OD values of the supernatants (100 µL) were then measured using a UV/VIS spectrophotometer (UNIC 2802S, China) at 545 nm. The hemolysis rate was calculated using the following formula:
Histocompatibility evaluation in subcutaneous injection model
Nine male Sprague‒Dawley rats (280–300 g) were used to evaluate the immune response and cell infiltration of different materials. 1 mL mECM hydrogel, PM (3 mg) in 1 mL saline and 1 mL mECM + PM composites were prepared under sterile conditions and loaded into a 2.5 mL syringe, respectively. Then, they were subcutaneously injected into rats for 1 and 4 weeks. H&E staining was used to assess cell infiltration. Masson staining was used to assess the collagen deposition and distribution. To evaluate the immune response, immunofluorescence staining was performed to identify the immune cells with the following antibodies: CD68 (1:250, Abcam, ab31630), iNOS (1:300, Abcam, ab15323), CD206 (1:300, Abcam, ab64693), CD45 (1:150, Abcam, ab10558), CD20 (1:100, Abcam, ab64088) and CD3 (1:100, Abcam, ab16669). Subsequently, the following secondary antibodies were reacted with the corresponding primary antibodies: goat anti-rabbit IgG Alexa 594 (1:500, Invitrogen, USA) and goat anti-mouse IgG1 Alexa 488 (1:500, Invitrogen, USA). DAPI staining was used to label the cell nucleus (Southern Biotech, England). Finally, images were observed using a confocal laser microscope (Leica, SP8, Germany). The S/L value of microspheres, cell infiltration, and fluorescence intensity of iNOS and CD206 were measured using Image-Pro Plus 6.0 software.
Construction of the rat VWL model
To prepare the VML models, adult male Sprague‒Dawley rats (aged 8–10 weeks with a weight range of 280–300 g were randomly assigned to the untreated (saline), PM, mECM, mECM + PM, mECM@IL4 + PM, mECM + PM@IGF1 and mECM@IL4 + PM@IGF1 composites groups. After anesthetization with ketamine (40 mg/kg)-xylazine (5 mg/kg)-acepromazine (1 mg/kg), and isoflurane gas was used as assisted anesthesia throughout the procedure. The hairs of rats’ right legs were removed, and the skin was cut to expose the tibialis anterior muscle. The muscle defect with 10 mm × 6 mm ×4 mm defect accounting for a volumetric loss of ~40% of the tibialis anterior was resected using the surgical blade. After implantation of different materials, the clinical 3 M breathable patch was fixed to the defect site using 9-0 sutures (Lingqiao, Ningbo, China). The skin was closed with 3–0 monofilament nylon sutures (Lingqiao, Ningbo, China). All surgical procedures were performed in SPF environment, and all rats were fasting 12 h before and after surgery, but can have water. Finally, rats were euthanized at 2 and 8 weeks, and the regenerated muscle was obtained for histological and immunofluorescence staining analysis.
Electrophysiology and blood flow recovery assessment
To characterize the electrophysiologic properties 8 weeks post-surgery, the rats were anesthetized, and the experimental muscle side was exposed. Then, stimulating electrodes combined with acupuncture needles were pierced into the two ends of the regenerated muscle, the compound muscle action potentials (CMAPs) were recorded using a 4-channel physiologic signal recorder (RM-6240, Chengdu Instrument Factory, Chengdu, China). The CMAP results were compared with the tibialis anterior muscle of the contralateral normal side.
Laser Doppler imaging was conducted to quantitatively detect blood flow recovery of neo-muscle at 8 weeks using a PeriCam PSI Information System (Perimed AB, Sweden). The sampling frequency was 60 Hz, and the sampling time was 25 frames per second. The average values of blood flow were subsequently determined.
Histological staining
Muscle histological analysis was performed at 2 and 8 weeks after surgery. Briefly, the regenerated muscles of the experimental side and normal side were picked after euthanasia. Then, the explants were fixed in 4% paraformaldehyde at 4 °C for one day, then embedded in paraffin, cut into 5 μm sections with a microtome (Leica, Germany) and placed in 65 °C ovens for 6 h.
Before histological staining, muscle tissue sections were placed in xylene for 40 min to remove paraffin, followed by hydration using gradients of alcohol and immersion in distilled water for 5 min. Then, H&E and Masson staining were performed according to the kit instructions. Images were captured using a Leica microscope (Leica DM3000, Germany). The integrated optical density (IOD) of collagen fibers per unit area (mm2) was measured using Image-Pro Plus 6.0 software.
Immunofluorescence staining
After dewaxing and gradient hydration of the tissue sections, antigen repair was performed using sodium citrate solution, followed by blocking the nonspecific binding sites with 5% goat serum for 40 min and then incubating with different antibodies overnight at 4 °C, including Desmin rabbit polyclonal antibody (1:50, Abcam, ab15200), α-SMA rabbit polyclonal antibody (1:300, Abcam, ab7817), NF-09 mouse monoclonal antibody (1:200, Abcam, ab7794), iNOS rabbit polyclonal antibody (1:200, Abcam, ab15323), TNF-α rabbit polyclonal antibody (1:100, Abcam, ab183218), and rabbit CD206 polyclonal antibody (1:500, Abcam, ab64693). After washing with PBS, the sections were incubated with the corresponding secondary antibodies for 60 min at 37 °C. After washing with PBS 3 times, the tissue sections were stained with DAPI. The fluorescent images of the sections were captured with a confocal laser microscope (Leica, SP8, Germany). The quantification of fluorescence intensity was assessed using Image-Pro Plus 6.0 software.
Statistical analysis
Each experiment was repeated at least three times independently with similar results. All data are expressed as the mean ± standard deviation (SD). GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analysis. Image pro-plus 6.0 software was used for semi-quantitative analysis of fluorescence intensity. At least three samples per group were randomly selected and measured in each group. Briefly, the single-channel fluorescence image was converted into a gray image at first, and the gray value of each pixel represents the fluorescence intensity. The software will select a threshold uniformly for measurement, and the integrated optical density (IOD) for specific area was quantified. For the measurement of microspheres diameter, pore size, and cell number, length, area, etc., the details of quantification were presented in main text. Single comparisons were performed using an unpaired Student’s t test. Multiple comparisons were performed using Tukey’s post hoc test and one-way analysis of variance (ANOVA). Multivariant comparisons were performed using two-way ANOVA with Tukey’s post hoc test. For all tests, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 indicate statistical significance, ns: no significance.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data needed to support the conclusions in the study are available within the article and/or the supplementary files and movies. Data underlying Figs. 1–9 and Supplementary Figs. 1–13 are provided with this paper in the source data file. Any additional requests for information can be directed to, and will be fulfilled by the corresponding authors. Source data files are provided with this paper. Source data are provided with this paper.
References
Gaharwar, A. K., Singh, I. & Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 5, 686–705 (2020).
Safina, I. & Embree, M. C. Biomaterials for recruiting and activating endogenous stem cells in situ tissue regeneration. Acta Biomater. 143, 26–38 (2022).
Anitua, E., Sanchez, M. & Orive, G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv. Drug Deliv. Rev. 62, 741–752 (2010).
Ashammakhi, N. et al. Minimally invasive and regenerative therapeutics. Adv. Mater. 31, e1804041 (2019).
Zhao, D. et al. Electrophoretic deposition of novel semi-permeable coatings on 3D-printed Ti-Nb alloy meshes for guided alveolar bone regeneration. Dental Mater. 38, 431–443 (2022).
Hussey, G. S., Dziki, J. L. & Badylak, S. F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 3, 159–173 (2018).
Wang, S. et al. Alkaline activation of endogenous latent TGFbeta1 by an injectable hydrogel directs cell homing for in situ complex tissue regeneration. Bioact. Mater. 15, 316–329 (2022).
Feng, Q. et al. Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials 101, 217–228 (2016).
Lin, F., Wang, Z., **ang, L., Deng, L. & Cui, W. Charge‐guided micro/nano‐hydrogel microsphere for penetrating cartilage matrix. Adv. Funct. Mater. 31, 2107678 (2021).
Xu, Y. et al. Metabolism balance regulation via antagonist‐functionalized injectable microsphere for nucleus pulposus regeneration. Adv. Funct. Mater. 30, 2006333 (2020).
Kankala, R. K. et al. Highly porous microcarriers for minimally invasive in situ skeletal muscle cell delivery. Small 15, e1901397 (2019).
Ungerleider, J. L. et al. Extracellular matrix hydrogel promotes tissue remodeling, arteriogenesis, and perfusion in a rat hindlimb ischemia model. JACC Basic Transl. Sci. 1, 32–44 (2016).
Hu, J. et al. Bioactive-tissue-derived nanocomposite hydrogel for permanent arterial embolization and enhanced vascular healing. Adv. Mater. 32, e2002611 (2020).
Traverse, J. H. et al. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl. Sci. 4, 659–669 (2019).
Diaz, M. D. et al. Injectable myocardial matrix hydrogel mitigates negative left ventricular remodeling in a chronic myocardial infarction model. JACC Basic Transl. Sci. 6, 350–361 (2021).
**ng, H. et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J. Nanobiotechnol. 19, 264 (2021).
Zhang, D. et al. Large fuzzy biodegradable polyester microspheres with dopamine deposition enhance cell adhesion and bone regeneration in vivo. Biomaterials 272, 120783 (2021).
Ventura, R. D., Padalhin, A. R., Kim, B., Park, M. & Lee, B. T. Evaluation of bone regeneration potential of injectable extracellular matrix (ECM) from porcine dermis loaded with biphasic calcium phosphate (BCP) powder. Mater. Sci. Eng. C Mater. Biol. Appl. 110, 110663 (2020).
Wei, D. X., Dao, J. W. & Chen, G. Q. A micro-ark for cells: highly open porous polyhydroxyalkanoate microspheres as injectable scaffolds for tissue regeneration. Adv. Mater. 30, e1802273 (2018).
Huang, C. C. et al. Injectable PLGA porous beads cellularized by hAFSCs for cellular cardiomyoplasty. Biomaterials 33, 4069–4077 (2012).
Bee, S.-L. et al. Approaches to improve therapeutic efficacy of biodegradable PLA/PLGA microspheres: a review. Poly. Rev. 58, 495–536 (2018).
Li, C. et al. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 5, 61–81 (2019).
Saldin, L. T., Cramer, M. C., Velankar, S. S., White, L. J. & Badylak, S. F. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 49, 1–15 (2017).
Tidball, J. G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 17, 165–178 (2017).
Chazaud, B. Inflammation and skeletal muscle regeneration: leave it to the macrophages! Trends Immunol. 41, 481–492 (2020).
Deng, Y. et al. Inflammation‐instructed hierarchical delivery of il‐4/mir‐21 orchestrates osteoimmune microenvironment toward the treatment of rheumatoid arthritis. Adv. Funct. Mater. 31, 2101033 (2021).
Hachim, D., LoPresti, S. T., Yates, C. C. & Brown, B. N. Shifts in macrophage phenotype at the biomaterial interface via il-4 eluting coatings are associated with improved implant integration. Biomaterials 112, 95–107 (2017).
**, S.-S. et al. A biomimetic hierarchical nanointerface orchestrates macrophage polarization and mesenchymal stem cell recruitment to promote endogenous bone regeneration. ACS Nano 13, 6581–6595 (2019).
Liu, S. et al. Immunomodulatory hybrid micro-nanofiber scaffolds enhance vascular regeneration. Bioact. Mater. 21, 464–482 (2023).
Bonito, V. et al. Distinct effects of heparin and interleukin-4 functionalization on macrophage polarization and in situ arterial tissue regeneration using resorbable supramolecular vascular grafts in rats. Adv. Healthc. Mater. 10, e2101103 (2021).
Zou, M., Sun, J. & **ang, Z. Induction of m2-type macrophage differentiation for bone defect repair via an interpenetration network hydrogel with a go-based controlled release system. advanced healthcare. Materials 10, e2001502 (2021).
Li, Y. et al. Ultrasound controlled anti-inflammatory polarization of platelet decorated microglia for targeted ischemic stroke therapy. Angew. Chem. Int. Ed. 60, 5083–5090 (2021).
Raimondo, T. M. & Mooney, D. J. Anti-inflammatory nanoparticles significantly improve muscle function in a murine model of advanced muscular dystrophy. Sci. Adv. 7, eabh3693 (2021).
Qiu, T. et al. IGF-I induced phosphorylation of PTH receptor enhances osteoblast to osteocyte transition. Bone Res. 6, 5 (2018).
Park, J. et al. Oral delivery of novel human IGF-1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing. Biomaterials 233, 119591 (2020).
Hanwright, P. J. et al. Sustained IGF-1 delivery ameliorates effects of chronic denervation and improves functional recovery after peripheral nerve injury and repair. Biomaterials 280, 121244 (2022).
Geiger, B. C., Wang, S., Padera, R. F. Jr., Grodzinsky, A. J. & Hammond, P. T. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 10, eaat8800 (2018).
Mourkioti, F. & Rosenthal, N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 26, 535–542 (2005).
Raimondo, T. M. et al. Combined delivery of VEGF and IGF-1 promotes functional innervation in mice and improves muscle transplantation in rabbits. Biomaterials 216, 119246 (2019).
Shah, N. J. et al. Synthetic nanoscale electrostatic particles as growth factor carriers for cartilage repair. Bioeng. Transl. Med. 1, 347–356 (2016).
Zhu, M. et al. Biodegradable and elastomeric vascular grafts enable vascular remodeling. Biomaterials 183, 306–318 (2018).
Syed, O., Walters, N. J., Day, R. M., Kim, H. W. & Knowles, J. C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 10, 5043–5054 (2014).
Gilbert-Honick, J. & Grayson, W. Vascularized and innervated skeletal muscle tissue engineering. Adv. Healthc. Mater. 9, e1900626 (2020).
Alarcon-Martinez, L. et al. Capillary pericytes express alpha-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife 7, e34861 (2018).
Spang, M. T. & Christman, K. L. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta Biomater. 68, 1–14 (2018).
Zhou, Z., Wu, W., Fang, J. & Yin, J. Polymer-based porous microcarriers as cell delivery systems for applications in bone and cartilage tissue engineering. Int. Mater. Rev. 66, 77–113 (2021).
Lu, S. et al. Polydopamine-decorated PLCL conduit to induce synergetic effect of electrical stimulation and topological morphology for peripheral nerve regeneration. Small Methods 7, e2200883 (2023).
Lorden, E. R. et al. Mitigation of hypertrophic scar contraction via an elastomeric biodegradable scaffold. Biomaterials 43, 61–70 (2015).
Ji, X. et al. Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration. Biomaterials 285, 121530 (2022).
Li, J. et al. Articular fibrocartilage-targeted therapy by microtubule stabilization. Sci. Adv. 8, eabn8420 (2022).
Li, Q., Chang, B., Dong, H. & Liu, X. Functional microspheres for tissue regeneration. Bioact. Mater. 25, 485–499 (2023).
Nakayama, K. H., Shayan, M. & Huang, N. F. Engineering biomimetic materials for skeletal muscle repair and regeneration. Adv. Healthc. Mater. 8, e1801168 (2019).
Dziki, J. et al. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. Npj Regen. Med. 1, 16008 (2016).
Juhas, M. et al. Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration. Nat. Biomed. Eng. 2, 942–954 (2018).
Sadtler, K. et al. Develo** a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366–370 (2016).
Lokwani, R. et al. Pro-regenerative biomaterials recruit immunoregulatory dendritic cells after traumatic injury. Nature Materials (2023).
Ferreira, R. et al. Sugar or fat: the metabolic choice of the trained heart. Metabolism 87, 98–104 (2018).
Grunseich, C. et al. Safety, tolerability, and preliminary efficacy of an IGF-1 mimetic in patients with spinal and bulbar muscular atrophy: a randomised, placebo-controlled trial. Lancet Neurol. 17, 1043–1052 (2018).
Rommel, C. et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3, 1009–1013 (2001).
Glass, D. J. Molecular mechanisms modulating muscle mass. Trends Mol. Med. 9, 344–350 (2003).
Yoshida, T. & Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 9, 1970 (2020).
Shi, J. et al. Regulation of the inflammatory response by vascular grafts modified with aspirin-triggered resolvin D1 promotes blood vessel regeneration. Acta Biomater. 97, 360–373 (2019).
Zhang, J. et al. Endothelial lactate controls muscle regeneration from ischemia by inducing m2-like macrophage polarization. Cell Metab. 31, 1136–1153.e1137 (2020).
Li, X. et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials 245, 119978 (2020).
Liu, Y. et al. PLGA hybrid porous microspheres as human periodontal ligament stem cell delivery carriers for periodontal regeneration. Chem. Eng. J. 420, 129703 (2021).
Zhu, M. et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 10, 4620 (2019).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) projects (81921004 (D.K.), 82272156 (M.Z.), 82202775 (W.L.)), CAMS Innovation Fund for Medical Sciences (no. 2022-I2M-1-023 (M.Z.)), the China Postdoctoral Science Foundation (BSMS7100011 (W.L.)). Inner Mongolia Autonomous Region Science and Technology Project (2022YFSH0063 (J.Z.)) and Bei**g-Tian**-Hebei Basic Research Cooperation Special Project (22JCZXJC00100 (M.Z.)).
Author information
Authors and Affiliations
Contributions
M.Z. and D.K. conceived the research; Y.L., M.Z., D.K., and W.L. designed the experiments; Y.L, S.L. and Y.W., performed the experiments; Y.L, S.L., Y.W., H.L., F.N., and Y.S., designed, fabricated and characterized composites; Y.L, J.Z., S.L., Y.W., and G.S., performed microsurgery of animal experiments. Y.L., M.Z., A.C.M., D.K., W.L., and Y.Z., interpreted the data, analysed the data and wrote the manuscript. All authors discussed the data and direction of the project at regular intervals throughout the study.
Corresponding authors
Ethics declarations
Competing interests
All authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Theresa Raimondo, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Liu, S., Zhang, J. et al. Elastic porous microspheres/extracellular matrix hydrogel injectable composites releasing dual bio-factors enable tissue regeneration. Nat Commun 15, 1377 (2024). https://doi.org/10.1038/s41467-024-45764-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45764-4
- Springer Nature Limited