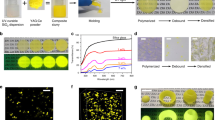
Abstract
Phosphor-glass composites (PGC) are excellent candidates for highly efficient and stable photonic converters; however, their synthesis generally requires harsh procedures and long time, resulting in additional performance loss and energy consumption. Here we develop a rapid synthetic route to PGC within about 10 seconds, which enables uniform dispersion of Y3Al5O12:Ce3+ (YAG:Ce) phosphor particles through a particle self-stabilization model in molten tellurite glass. Thanks for good wettability between YAG:Ce micro-particles and tellurite glass melt, it creates an energy barrier of 6.94 × 105 zJ to prevent atomic-scale contact and sintering of particles in the melt. This in turn allows the generation of YAG:Ce-based PGC as attractive emitters with high quantum efficiency (98.4%) and absorption coefficient (86.8%) that can produce bright white light with luminous flux of 1227 lm and luminous efficiency of 276 lm W−1 under blue laser driving. This work shows a generalizable synthetic strategy for the development of functional glass composites.
Similar content being viewed by others
Introduction
Rare earth phosphor materials as photonic converters, exampled by commercial Y3Al5O12:Ce3+(YAG:Ce), CaAlSiN3:Eu2+, and Sr[LiAl3N4]:Eu2+, etc., have received broad attentions in lighting and display1,2,Full size image
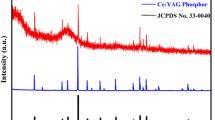
To investigate the interfacial stability between YAG:Ce particles and tellurite glass, we firstly measured the X-ray diffraction (XRD) patterns of YAG:Ce-PGC. Obviously, all diffraction peaks are coincident to YAG phase suggesting that the incorporation of YAG:Ce did not induce the precipitation of other crystalline phases (Supplementary Fig. 6). Subsequently, the local structures of YAG:Ce-PGC were evaluated by Raman vibrational modes (Supplementary Fig. 7). The Raman spectra of the YAG:Ce-PGC fabricated at 650 °C, with a superposition of those from tellurite glass and YAG:Ce, represents a pivotal signal that the interface reaction is absent during rapid synthesis. The interfacial stability of YAG:Ce particles dispersed in tellurite glass at nanometer scale was further characterized by high-resolution transmission electron microscopy (HRTEM), selected area electron diffraction (SAED), and energy dispersive spectroscopy (EDS) map** analysis. As illustrated, a boundary line between crystal and amorphous phases is explicit, and HRTEM images and SAED patterns confirm the cubic garnet phase of YAG:Ce and the amorphous phase of tellurite glass (Supplementary Fig. 8). From the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image, a well-defined boundary can be also observed, which is manifested by the EDS map** profile, showing significant elemental differences and no observable element diffusion region between the YAG:Ce crystal and tellurite glass (Fig. 1g). These results indicate that interfacial interactions between YAG:Ce particles and tellurite glass are negligible, which is in stark contrast to the thickness of about 50–300 nm in the previous studies13,26,36,37, and it further proves that our synthesis method is a nondestructive composite strategy.
Verification of particle self-stabilization model
To investigate the dispersion stability of YAG:Ce particles in tellurite glass melt, we put the glass melt with uniformly dispersed YAG:Ce particles back into the high-temperature furnace to continue heating at 650 °C and took it out at different holding times, quenched, and formed. The YAG:Ce particles were uniformly dispersed even after our samples remained in the liquid state for about 120 s without agitation processing (Supplementary Fig. 9). This provides convincing evidence that YAG:Ce particles realize good dispersion and self-stabilization in molten tellurite glass before solidification. To better understand the physical process, a theoretical analysis was performed, and the details were given in the methods section on the particle self-stabilization model. The results show that the particle self-stabilization is ascribed to the synergy of the following three main factors, as schematically shown in Fig. 2a: (1) At 650 °C, the small wetting angle between YAG:Ce particles and the molten tellurite glass is 43.5°, which promotes the formation of the new interface with an energy barrier of about 6.94 × 105 zJ, preventing the atomic-scale contact and sintering of YAG:Ce particles in the melt. (2) A attractive van der Waals potential between the YAG:Ce particles in the tellurite glass melt, about −2.42 × 105 zJ at the secondary minimum of Fig. 2a, inducing particle aggregation. (3) A weak thermal energy of about 12.74 zJ helps to drive YAG:Ce particle dispersion.
a Potential energy diagram of YAG:Ce particles self-stabilization. Wbarrier is the interfacial energy barrier between tellurite glass and YAG:Ce particle; Wthermal is the thermal energy; WvdW (min) is the van der Waals potential for maximum attraction between two YAG:Ce particles. Segment 1 is attributed to van der Waals interaction potential energy, which induces particle aggregation; segment 2 is dominated by the interfacial energy barrier, which resists the contact of YAG:Ce particles in glass melt; segment 3 is the interfacial energy barrier descent owing to the tellurite glass-YAG:Ce interface being replaced by YAG:Ce interface. The inset is the wetting angle photograph between tellurite glass melt and YAG:Ce ceramics. b Schematic diagram of uniform dispersion and self-stabilization of YAG:Ce particles in tellurite glass melt. The YAG:Ce particles were poured into the low-viscosity and high surface energy tellurite glass melt. The glass melt quickly wrapped the surface of the YAG:Ce particles and formed new interface with high energy barrier, which prevented YAG:Ce particles from contact and sintering based on the self-stabilization model of YAG particles. c Time-dependent fluent simulation of YAG particles dispersed in tellurite glass melt (at different stages of 0, 1, 3 and 5 s) under the agitation speed of 5 revolutions per second (rev s−1), and the color depth of the particle represents the velocity at that moment. Images used courtesy of ANSYS, Inc.
When we pour the YAG:Ce particles into the low-viscosity tellurite glass melt, the YAG:Ce particles are rapidly dispersed in the melt under the agitation drive of the quartz glass rod and the heat drive (Fig. 2b). Simultaneously, the glass melt quickly wraps the surface of YAG:Ce particles owing to the good wettability between tellurite glass melt and YAG:Ce particles, and forms new interface layers with high energy barrier. The repulsive energy barrier to prevent YAG:Ce particles from contact and sintering, which is the main driving force behind the self-stabilization of YAG particles in the tellurite glass melt, is much higher than attractive van der Waals potential (Fig. 2a). Consequently, the synergy of high repulsive energy barrier and heat energy make the YAG:Ce particles break free from the quasi-clusters formed by van der Waals potential attraction, resulting in dispersed particles in the melt. This particle self-stabilization model allows to create a uniform dispersion of dense particles in liquids and providing more possibilities for functional composites when the repulsive force cannot be generated by conventional techniques. The visualization of intermediate state and final state for YAG: Ce particles in tellurite glass melt by simple agitation, which is helpful to understand the dynamic process of particle dispersion. We utilized the shear stress transport k-omega turbulence model and the discrete phase model in ANSYS Fluent to simulate the flow characteristics of the fluid and the motion state of the particles38,39. The particles in the crucible container were dispersed downward in a spiral shape under the different agitation speed to 1, 3, and 5 rev s−1 (Fig. 2c and Supplementary Fig. 10). When the agitation speed is increased to 5 rev s−1 for 3–5 s, the particles are uniformly filled throughout the fluid, and the velocity of the particles is almost the same. The simulation results fully demonstrate that the complete dispersion of YAG:Ce particles can be achieved only by using simple agitation strategy under the low viscosity characteristic of tellurite glass.
Performance characterizations of YAG:Ce-PGC
Thanks for stable particle interface and uniform dispersion of YAG:Ce phosphor particles in tellurite glass, the YAG:Ce-based PGC can be used as an attractive photonic converter. As depicted in Fig. 3a, the photoluminescence excitation (PLE) spectra of YAG:Ce-PGC consist of two excitation bands centered at 343 nm and 450 nm, respectively. Under 450 nm blue light excitation, the 5d → 4 f transition of Ce3+ brings a broad yellow emission with the center wavelength of 552 nm40. However, the weak excitation peak at 343 nm in YAG:Ce-PGC contributes to the high absorption of the tellurite glass in the ultraviolet range (Fig. 1d). The PLE and PL spectra of YAG:Ce-PGC exhibit similar profiles to YAG:Ce powder, demonstrating that the embedded YAG:Ce particles are the active components of YAG:Ce-PGC. Similarly, the PL decay curves of YAG:Ce-PGC and YAG:Ce powder overlap almost completely (Supplementary Fig. 11). The unchanged steady-state and transient PL properties of YAG:Ce further confirm the negligible interface reaction between YAG:Ce and tellurite glass. As portrayed in Fig. 3b, the IQE of all samples is no less than 92%, with an absorption of no less than 85% (Supplementary Table 2). The high quantum efficiency and absorption are put down to the fact that the undamaged YAG:Ce particles are densely and uniformly dispersed in the tellurite glass matrix, producing high-efficient light scattering. Moreover, YAG:Ce-PGC 20 wt% sample possesses thermal conductivities of 1.52 and 1.78 W m−1 K−1 at ambient temperature and 250 °C, respectively, which is seven times that of organic resins (0.2 W m−1 K−1) and comparable to silica glass (1.49 W m−1 K−1) (Supplementary Fig. 12)25. The relatively high thermal conductivity also facilitates the application of high-power light sources. The proposed strategy is a general way for the development of various PGC. To verify this, diverse translucent PGC samples have been synthesized by replacing YAG:Ce with LuAG:Ce and GdAG:Ce (denoted as LuAG:Ce-PGC and GdAG:Ce-PGC, respectively). As expected, all the as-synthesized PGC are highly efficient with limited IQE loss (Supplementary Fig. 13).
a PLE and PL spectra of YAG:Ce-PGC and YAG:Ce powder. b Internal/external quantum efficiency (I/EQE) and absorption efficiency (AE) of YAG:Ce-PGC. IQE (AE) of YAG:Ce powder was determined to be 99.4% (74.5%). c Schematic diagram of laser driven light source test system. A 450 nm blue laser is emitted from the laser source and irradiated onto the sample using two optical focusing mirror. The light emitted by the sample and the reflected blue laser are then collected using an integrating sphere and analyzed within the fiber optic spectrometer. d Luminous flux and e the luminous efficacy of YAG:Ce-PGC with different concentrations as a function of the laser power density. f Photostability of the YAG:Ce-PGC, commercial YAG:Ce phosphor in glass (YAG:Ce-PiG), and YAG:Ce phosphor in silicone resin (YAG:Ce-PiS) under 2 W mm−2 blue laser irradiation. Source data are provided as a Source Data file.
To evaluate the potential application of laser-driven white light illumination, the photoelectric properties of YAG:Ce-PGC were characterized (Fig. 3c). Under the excitation of focused blue laser, the luminous flux (LF) initially showed a linear increase with the incident laser power, reaching its peak value and then sharply decreased (Fig. 3d). This emission saturation at high power density is commonly observed in laser-driven illumination, attributed to the accumulated heat in the color converter and subsequent PL thermal quenching33. With increasing do** concentration, the saturation threshold of the YAG:Ce-PGC sample first increased and then decreased, almost the same as that of the sample temperature (Supplementary Fig. 14), which indicates the higher the concentration of YAG:Ce phosphor, the more blue lasers may be down-converted in the same radiation region, generating more heat and accelerating the luminous saturation of the sample26. Prior to the occurrence of emission saturation, the luminous efficiency gradually decreased (Fig. 3e), and the maximum values of luminous flux, luminous efficiency, and the laser saturation threshold are 1227 lm, 276 lm W−1 mm−1, 8.5 W mm−1, respectively. It is found that the as-measured parameters are superior to commercial YAG:Ce phosphor in glass (YAG:Ce-PiG), and the detailed composition is shown in Supplementary Fig. 15 and Supplementary Table 3. The corresponding CRI and CCT decreased with increasing do** concentration (Supplementary Fig. 16). The CRI and CCT of the PGC samples with YAG:Ce do** concentration between 10 wt% and 15 wt% show a sudden drop, attributed to the synergy of increase in YAG:Ce content and porous characters (Supplementary Fig. 17). The thermal performance of the color converter is crucial in light source applications. As shown in the supplementary Fig. 18, the integrated emission intensity of YAG:Ce-PGC at 150 °C can still maintain 96% of that at 30 °C, and is also superior to that of YAG:Ce phosphor in silicone resin (YAG:Ce-PiS) and YAG:Ce powder. In addition, the operational stability of composites was tested under different encapsulation strategies under continuous 2 W mm−2 blue laser irradiation. As plotted in Fig. 3f, YAG:Ce-PGC exhibited comparable operation stability as the commercial YAG:Ce-PiG under continuous 7.5 h blue laser irradiation, while the YAG:Ce-PiS was irreversibly destroyed merely within 30 s at the same power. These excellent optical performance and stability undoubtedly validate that the rapid synthesis strategy of YAG:Ce fully encapsulated in dense tellurite glass can not only achieve phosphor particle integrity, but also effectively protect YAG:Ce phosphor from oxygen, moisture, light exposure, and heat damage.
In summary, we propose a facile and fast agitation synthetic protocol in seconds for phosphor-glass composites with stable particle interface and dense uniform dispersion. Theoretical and experimental studies reveal that the dense uniform dispersion of phosphor particles in tellurite glass originates from the good wettability between YAG:Ce particles and the tellurite glass melt, which creates an energy barrier to prevent particle clustering in the melt. The nondestructive interface of phosphor and tellurite glass can be emanated in local structures and confirmed by the improved luminescence properties. The resultant YAG:Ce-based PGC possess high quantum efficiency of 98.4% and absorption coefficient of 86.8%, presenting great potential for high-power white lighting. In addition, the universality of the strategy is confirmed, and different color-tunable translucent composites are also prepared. This work provides a straightforward and general synthetic protocol to functional glass composites for various light-emitting and light-detecting applications, and further expands the scope of imagination for the design and practical application of glass based composite materials with high stability.