Abstract
Controlling the selectivity of the electrocatalytic reduction of carbon dioxide into value-added chemicals continues to be a major challenge. Bulk and surface lattice strain in nanostructured electrocatalysts affect catalytic activity and selectivity. Here, we unravel the complex dynamics of synergistic lattice strain and stability effects of Cu-Ag tandem catalysts through a previously unexplored combination of in situ nanofocused X-ray absorption spectroscopy and Bragg coherent diffraction imaging. Three-dimensional strain maps reveal the lattice dynamics inside individual nanoparticles as a function of applied potential and product yields. Dynamic relations between strain, redox state, catalytic activity and selectivity are derived. Moderate Ag contents effectively reduce the competing evolution of H2 and, concomitantly, lead to an enhanced corrosion stability. Findings from this study evidence the power of advanced nanofocused spectroscopy techniques to provide new insights into the chemistry and structure of nanostructured catalysts.
Similar content being viewed by others
Introduction
The capture and subsequent electrocatalytic conversion of CO2 gas (eCO2RR) into value-added products, such as fuels, syngas, or alcohols, using renewable electricity represents one of the most attractive routes to establish a sustainable, circular economy, and mitigate anthropogenic CO2 emissions1,2g, h). In addition, improved morphological stability for Ag-modified Cu catalyst NPs compared to monometallic Cu NPs (Fig. 2e, f and Supplementary Figs. 4–6) can be claimed. XRD analysis (Fig. 2i and Supplementary Fig. 5) provides further evidence for the formation of phase-segregated domains of pure Cu and Ag in the tandem catalyst, which can be attributed to the positive enthalpy of Cu-Ag solid solutions5,14.
a Schematic illustration of the developed synthesis route for isolated Cu-Ag NPs on an electrically conductive GC substrate. b Representative ADF-STEM-EDX map** of a single, bimetallic NP (Cu0.88Ag0.12 catalyst). c Corresponding ADF-STEM image revealing the Cu-Ag interface and d the elemental distribution obtained from the EDX line-scan (c). e, f Representative SEM images of Cu0.88Ag0.12 and g, h corresponding SEM-EDX elemental map**s before and after eCO2RR in an H-cell setup in CO2-saturated 0.1 M KHCO3 electrolyte at room temperature. i GI-XRD analysis of the spent Cu0.88Ag0.12 catalyst revealing the presence of segregated Cu0 and Ag0 phases, according to the corresponding reference patterns shown as blue and red vertical bars, respectively. Broad reflections indicated by dark gray asterisks can be attributed to the GC substrate. j Nanofocused X-ray characterization techniques for the identification of active sites via combined structural and chemical analyses under reaction conditions.
To assess the stability of the synthesized NPs, eCO2RR tests were conducted in a three-electrode H-cell setup. The Cu-Ag tandem catalysts were tested with a focus on selectivity and durability. Results revealed that monometallic Cu NPs undergo severe restructuring and partial dissolution/corrosion after 6 h eCO2RR, whereas bimetallic Cu-Ag catalysts show improved durability without any pronounced sintering or particle detachment from the electrode surface (Supplementary Figs. 4, 6). Beside a strong adhesion of the NPs to the substrate, the absence of pronounced coalescence or sintering processes is critical for meaningful investigations using nanofocused X-ray probe techniques (cf. Fig. 2j). Reaction-induced morphological changes were previously reported to alter the eCO2RR product selectivity, e.g., by variations in the surface atomic coordination number or the exposed facets5,15,16.
Note that comparative EDX analyses of the pristine and tested bimetallic electrocatalysts indicated not only a very similar particle morphology but also an almost identical bulk composition (Fig. 2g, h and Supplementary Fig. 6). Both Cu- and Ag-rich domains remain discernible after the chronoamperometric durability test. A comparable stabilization effect at a moderate cathodic bias (−0.8 VRHE) was observed in our previous contribution5 for Cu-Ag NPs synthesized via ligand-assisted electrophoresis. Importantly, findings from the present study indicate a stabilization effect of the NPs against coalescence and corrosion even for low Ag contents (see Supplementary Fig. 6). Obviously, undesirable dissolution-redeposition processes17—leading to particle growth at sufficiently cathodic bias—are restrained by the presence of predominantly isolated Ag domains in the NPs (cf. Fig. 2b).
Besides improved durability, Cu-Ag tandem catalysts reveal an enhanced eCO2RR selectivity toward C1 products, particularly CO and CH4 (Fig. 3a and Supplementary Fig. 7). The introduction of Ag species suppresses the kinetically favored HER side-reaction to a large extent, as previously reported in the literature18. The bimetallic nature is expected to affect both catalytic activity and selectivity, as described for mono-10,19 and bimetallic4,7 Cu-based eCO2RR catalysts before. As a first step, we establish in situ nanofocused XANES to track the chemical states of individual NPs. Advantageously, with a beam size of ~80 × 100 nm (H x V), hard X-ray nanoprobe techniques at the ID16B beamline of the European Synchrotron Radiation Facility (ESRF, Grenoble, France) provide a superior spatial resolution for time-resolved nano-XAS measurements under operating conditions. Figure 3b schematically illustrates a customized cell built for potential-dependent eCO2RR measurements in liquid electrolyte (see Supplementary Fig. 8 for further details). A previously established nano-XRF technique20,21 was used for the map** of the NPs. As depicted in Supplementary Fig. 9, Cu-specific elemental distribution maps were obtained from the acquired nano-XRF data with submicron resolution. Based on these elemental maps, the location-specific chemical structure can be resolved for an individual particle. At open circuit potential (OCP), the results imply a higher average oxidation state,. the presence of CuI/II species in close vicinity of the interface to the liquid electrolyte (see Supplementary Fig. 9)17,22. Evaluations of the acquired in situ XANES series unambiguously reveal the predominant existence of metallic Cu0 species under cathodic bias, as indicated by the Pourbaix diagram of Cu1,13. Here, it has to be mentioned that no conclusions about the exact composition or oxidation states at the surface of a particle can be drawn, which lies beyond the scope of this work and would require a more surface-sensitive technique. Nonetheless, XANES spectra collected at the particle rim show similar features to those from the center (Supplementary Figs. 10–13), not providing any clear evidence for oxidized bulk Cux+ species. Note that the stable presence of CuI species during eCO2RR remains a subject of debate and advanced concepts were recently proposed to control the ratio of Cu0/CuI species for an enhanced C2+ product selectivity23,24,25,26.
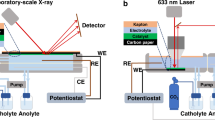
a Potential-dependent Faradaic efficiencies of Cu0.88Ag0.12 in eCO2RR in an H-cell setup in CO2-saturated 0.1 M KHCO3 electrolyte. b Schematic illustration of the developed nano-XAS setup at ID16B-ESRF beamline for the location- and potential-resolved acquisition of XANES data. c In situ Cu-K-edge XANES spectra (normalized) of individual Cu0.88Ag0.12 NPs during eCO2RR and d corresponding first derivatives (normalized). As a reference, a Cu0 metal foil was investigated in a dry state without any applied potential. Note that all potentials were converted to the RHE scale and 100% iR-compensated. Error bars in a represent standard deviations for triplicate experiments.
As a next step, in situ nano-BCDI experiments were carried out to track the atomic displacement distribution and strain (here, along the [002] direction) as a function of the applied potential in an individual NP (Figs. 4 and5 and Supplementary Fig. 14). For the evaluation of the obtained diffraction patterns (Fig. 4) and a reliable reconstruction of the particle’s shape, the 3D Bragg peak intensity is analyzed and complex variations in the electron density are required to be precisely recovered. The displacement field can be derived from the phase of the complex electronic density map of an individual NP. Computational efforts, e.g., iterative phase retrieval algorithms27,28, are utilized to reconstruct the particle shape as well as 3D strain maps29,30.
Representative diffraction patterns acquired at varying potentials on an individual Cu0.88Ag0.12 NP are illustrated. Red arrows highlight slight changes in the acquired fringe patterns observed under more negative potentials. Rocking curve (RC) parameters are given in the SI. Axis1 and axis2 correspond to the horizontal and vertical axes of the bi-dimensional detector, respectively. Note that all potentials were converted to the RHE scale and 100% iR-corrected.
a, b 2D, and c 3D reconstructions (real space) were obtained by applying a phase retrieval algorithm on the measured coherent X-ray diffraction pattern data. The size of the NP is ~450 nm. a, b the initial shape of the reconstructed crystal (at OCP) is shown as a guide to the eye (see black lines). Z is along the particle’s height, i.e., perpendicular to the substrate. The atomic displacement (u002) and the strain (\(\varepsilon\)002) along the [002] direction are displayed in a and in b–c, respectively. Note that all potentials were converted to the RHE scale and 100% iR-compensated.
Regarding the acquired potential-dependent diffraction patterns (Fig. 4), slight changes can be distinguished for the scan at −1.10 VRHE as well as the subsequent scan at OCP. Even though variations in the averaged lattice constants are below the resolution limit (<10−3%), we discover slight variations in the structure of the NP for negative potentials of ~−1.10 VRHE. Importantly, this effect seems to be irreversible, as the fringe pattern at the subsequent OCP step remains distorted.
Note that profound investigations of multiple NPs on the electrodes revealed the presence of different crystal orientations, with the majority of NPs exhibiting (111) and (002) orientations. Figure 5a illustrates the potential-dependent local deformation field inside an individual NP. The displacement field projection (Fig. 5a) and the local strain maps (Fig. 5b) along the [002] direction highlight the presence of tensile strain at the top and the bottom (i.e., at the interface to the GC substrate) of the crystal. At the center, contrarily, pronounced compressive strain is found. Between −0.85 and −1.00 VRHE, the overall shape of the crystal remains essentially unaltered. Yet, compared to the initial reconstructed crystal, an increase in compressive and tensile strain is found at the center and the top, respectively (Fig. 5b). Between −1.05 and −1.10 VRHE, a contraction in particle height, i.e., perpendicular to the substrate, can be observed (Fig. 5a, b, top/bottom). Beyond that, notch formation featuring reduced tensile strain is evident at the interface to the substrate at −1.05 VRHE (Fig. 5c, top/bottom). Concomitantly, a reduced tensile strain is found at the top of the crystal (Fig. 5b, top/bottom). At −1.10 VRHE, tensile strain increases at the top and the overall shape appears stretched along the x-axis. Tensile strain is predominant in the vicinity of the potential-induced notches at both the top and the bottom of the crystal, where the formation of local stress maxima is evident (see Fig. 5b, middle/bottom). Both the diffraction pattern (Fig. 4) and the strain map (Fig. 5b, c) derived from the last scan at OCP differ slightly from that of the initial state. Most notably, a diagonal strain propagation towards the surface of the crystal can be observed. Beyond that, the crystal irreversibly changed in overall shape and height.
Discussion
Ligand-free, bimetallic Cu-Ag NPs serve as robust model eCO2RR catalysts for the assessment of product selectivity and active site(s) identification. As a poor HER catalyst, the presence of low amounts of Ag atoms is found to effectively reduce competing H2 formation, which can be attributed to its larger free energy of adsorption relative to that of Cu18. Using hard X-ray-based nanoprobe techniques imposes strict requirements on the electrochemical setup and catalysts/electrodes (see Supplementary Figs. 8 and 14 for further information). The combination of nano-XRF and nano-XAS represents an emerging combination of advanced methodologies to probe local differences in the average oxidation state of an electrocatalyst and distinguish between a specific vs. an ensemble response. We note that the obtained information mainly originates from the bulk of the nanometer-scale particles. Clark and co-workers4 claimed the formation of Cu-Ag surface alloys with low Ag content and an enhanced selectivity toward oxygenates. Compressively-strained, less oxophilic Cu surface species were hypothesized. Rejecting the CO spillover concept, the authors conjectured surface strain as the main parameter to tune selectivity and, more generally, to explain the synergistic effects between Cu and Ag. The present contribution now provides direct evidence for three-dimensional atomic displacement/strain effects in bimetallic model catalysts with distinct Cu and Ag domains.
Nano-BCDI offers valuable information about the intraparticle strain distribution31. Currently, there are only a few reports about in situ BCDI studies. Recent advances improved the resolution as well as the coherent photon flux, which favorably reduced the acquisition time30. Taking advantage of the fourth generation Extremely Brilliant Source of the ESRF and a nanoprobe end-station, detailed insights into the dynamics of strain evolution of nanomaterials can be obtained32. Recently, a detailed study33 proposed a methodology for a reliable, quantitative analysis of displacement field and strain in nanocrystals. As illustrated in Fig. 5c, the maximum intraparticle strain along the [002] direction stayed within ±0.1% over the entire investigated potential range. Ex situ EDX elemental (Fig. 2g, h and Supplementary Figs. 2 and 3) and XRD analyses (Fig. 2i and Supplementary Fig. 5) evidence a segregated structure of Cu and Ag domains. Note that a polycrystalline character is predominant, as indicated by TEM/SAED analysis (Supplementary Fig. 3). Contrary to the evaluation of lattice strain by XRD analysis (ensemble response), nano-BCDI provides spatially resolved information (specific response) with a significantly improved accuracy, particularly for NP-based electrodes.
A few durable, Ag-rich NPs are present in both Cu0.88Ag0.12 and Cu0.95Ag0.05 samples (see Supplementary Fig. 6), which can be rationalized based on the Cu-Ag phase diagram34. These Ag-rich NPs, however, could not be properly analyzed via nano-XAS (see Supplementary Fig. 9 for further information). An averaged lattice constant of 4.074 Å was derived from the nano-BCDI data, which, according to Vegard’s law, indicates an Ag content of ~97 at-% for these few Ag-rich NPs with (002) orientation. Overall, sample stability remains the main challenge for a reliable in situ nano-BCDI evaluation under eCO2RR conditions. In this context, Cu0.88Ag0.12-based electrodes feature improved particle stability under X-ray irradiation, enabling full reconstruction of an individual crystal (Fig. 5).
In brief, for Cu0.88Ag0.12-based electrodes, most NPs reveal a robust, Janus-type structure with distinct metal domains and fully reduced (i.e., metallic) chemical states (cf. Supplementary Fig. 13), leading not only to an improved product selectivity but also to an enhanced durability. At about −0.95 VRHE, an increased CO Faradaic efficiency (FE) is found, which can either be attributed to the presence of the Ag domain or to the intraparticle Cu-Ag interface (see Supplementary Fig. 7)5,42.
For both synchrotron techniques, a custom-made in situ cell was used. Freshly prepared CO2-saturated 0.1 M KHCO3 electrolyte solution was continuously pumped through the setup (1 mL min−1). The working electrode (WE) was located at the center of the cell, which was sealed using a thin (~6 µm) Mylar film. For an optimized cell pressure, the height of the electrolyte reservoir relative to the mounted cell was adjusted. During the in situ experiments under negative potentials, the formation of large gas bubbles was observed at the electrode’s surface. To mitigate extensive bubble formation, OCP periods in the range of several minutes were inserted after each potential step. Note that both the formation and the removal of large gas bubbles were found to interfere with the acquisition of the XAS data.
Data availability
All data supporting the results of this study are included in the published article or the associated Supplementary Information.
References
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Sharifian, R., Wagterveld, R. M., Digdaya, I. A., **ang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814 (2021).
Wang, Q., Luo, J., Zhong, Z. & Borgna, A. CO2 capture by solid adsorbents and their applications: current status and new trends. Energy Environ. Sci. 4, 42–55 (2011).
Clark, E. L., Hahn, C., Jaramillo, T. F. & Bell, A. T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 139, 15848–15857 (2017).
Wu, L. et al. Stabilization effects in binary colloidal Cu and Ag nanoparticle electrodes under electrochemical CO2 reduction conditions. Nanoscale 13, 4835–4844 (2021).
Lai, Y. et al. Breaking scaling relationships in CO2 reduction on copper alloys with organic additives. ACS Cent. Sci. 7, 1756–1762 (2021).
Jansonius, R. P., Reid, L. M., Virca, C. N. & Berlinguette, C. P. Strain engineering electrocatalysts for selective CO2 reduction. ACS Energy Lett. 4, 980–986 (2019).
Kuhl, K. P. et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 136, 14107–14113 (2014).
Du, M., Zhao, X., Zhu, G., Hsu, H.-Y. & Liu, F. Elastic strain controlling the activity and selectivity of CO2 electroreduction on Cu overlayers. J. Mater. Chem. A 9, 4933–4944 (2021).
Kim, T., Kumar, R. E., Brock, J. A., Fullerton, E. E. & Fenning, D. P. How strain alters CO2 electroreduction on model Cu(001) surfaces. ACS Catal. 11, 6662–6671 (2021).
Liu, F., Wu, C. & Yang, S. Strain and ligand effects on CO2 reduction reactions over Cu–metal heterostructure catalysts. J. Phys. Chem. C. 121, 22139–22146 (2017).
Monzó, J. et al. Enhanced electrocatalytic activity of Au@Cu core@shell nanoparticles towards CO2 reduction. J. Mater. Chem. A 3, 23690–23698 (2015).
Lei, Q. et al. Structural evolution and strain generation of derived-Cu catalysts during CO2 electroreduction. Nat. Commun. 13, 4857 (2022).
Subramanian, P. R. & Perepezko, J. H. The ag-cu (silver-copper) system. J. Phase Equilibria 14, 62–75 (1993).
Reske, R., Mistry, H., Behafarid, F., Roldan Cuenya, B. & Strasser, P. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles. J. Am. Chem. Soc. 136, 6978–6986 (2014).
Gawande, M. B. et al. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev. 116, 3722–3811 (2016).
Vavra, J., Shen, T.-H., Stoian, D., Tileli, V. & Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem. Int. Ed. 60, 1347–1354 (2021).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23 (2005).
Sandberg, R. B., Montoya, J. H., Chan, K. & Nørskov, J. K. CO-CO coupling on Cu facets: coverage, strain and field effects. Surf. Sci. 654, 56–62 (2016).
Plass, C. T. et al. Spatially resolved dynamics of cobalt color centers in ZnO nanowires. Adv. Sci. 10, 2205304 (2023).
Martínez-Criado, G. et al. ID16B: a hard X-ray nanoprobe beamline at the ESRF for nano-analysis. J. Synchrotron Radiat. 23, 344–352 (2016).
Lee, S. H. et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ X-ray characterization. J. Am. Chem. Soc. 143, 588–592 (2021).
Liang, Z.-Q. et al. Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2. Nat. Commun. 9, 3828 (2018).
Lum, Y. & Ager, J. W. Evidence for product-specific active sites on oxide-derived Cu catalysts for electrochemical CO2 reduction. Nat. Catal. 2, 86–93 (2019).
Timoshenko, J. et al. Steering the structure and selectivity of CO2 electroreduction catalysts by potential pulses. Nat. Catal. 5, 259–267 (2022).
Arán-Ais, R. M., Scholten, F., Kunze, S., Rizo, R. & Roldan Cuenya, B. The role of in situ generated morphological motifs and Cu(i) species in C2+ product selectivity during CO2 pulsed electroreduction. Nat. Energy 5, 317–325 (2020).
Favre-Nicolin, V. et al. PyNX: high-performance computing toolkit for coherent X-ray imaging based on operators. J. Appl. Crystallogr. 53, 1404–1413 (2020).
Fienup, J. R. Reconstruction of an object from the modulus of its Fourier transform. Opt. Lett. 3, 27–29 (1978).
Karpov, D. & Fohtung, E. Bragg coherent diffractive imaging of strain at the nanoscale. J. Appl. Phys. 125, 121101 (2019).
Vicente, R. A., Neckel, I. T., Sankaranarayanan, S. K. R. S., Solla-Gullon, J. & Fernández, P. S. Bragg coherent diffraction imaging for in situ studies in electrocatalysis. ACS Nano 15, 6129–6146 (2021).
Kawaguchi, T. et al. Electrochemically induced strain evolution in Pt–Ni alloy nanoparticles observed by bragg coherent diffraction imaging. Nano Lett. 21, 5945–5951 (2021).
Atlan, C. et al. Imaging the strain evolution of a platinum nanoparticle under electrochemical control. Nat. Mater. 22, 754–761 (2023).
Carnis, J. et al. Towards a quantitative determination of strain in Bragg coherent X-ray diffraction Imaging: artefacts and sign convention in reconstructions. Sci. Rep. 9, 17357 (2019).
Jabbareh, M. A. & Monji, F. Thermodynamic modeling of Ag – Cu nanoalloy phase diagram. Calphad 60, 208–213 (2018).
Ma, M., Trześniewski, B. J., **e, J. & Smith, W. A. Selective and efficient reduction of carbon dioxide to carbon monoxide on oxide-derived nanostructured silver electrocatalysts. Angew. Chem. Int. Ed. 55, 9748–9752 (2016).
Wang, J. et al. Silver/copper interface for relay electroreduction of carbon dioxide to ethylene. ACS Appl. Mater. Interfaces 11, 2763–2767 (2019).
Huang, J., Mensi, M., Oveisi, E., Mantella, V. & Buonsanti, R. Structural sensitivities in bimetallic catalysts for electrochemical CO2 reduction revealed by Ag–Cu nanodimers. J. Am. Chem. Soc. 141, 2490–2499 (2019).
Huang, X. et al. Simplifying the creation of dumbbell-like Cu-Ag nanostructures and their enhanced catalytic activity. Chem. Eur. J. 18, 9505–9510 (2012).
Choi, C. et al. Intimate atomic Cu-Ag interfaces for high CO2RR selectivity towards CH4 at low over potential. Nano Res. 14, 3497–3501 (2021).
Liang, L. et al. Electroreduction of CO2 on Au(310)@Cu high-index facets. Angew. Chem. Int. Ed. 62, e202218039 (2023).
Sanchez-Cano, C., Gianolio, D., Romero-Canelon, I., Tucoulou, R. & Sadler, P. J. Nanofocused synchrotron X-ray absorption studies of the intracellular redox state of an organometallic complex in cancer cells. Chem. Commun. 55, 7065–7068 (2019).
Atlan C., Chatelier C., Olson K. A python package to help coherent diffraction imaging (CDI) practitioners in their analysis (v.0.1.4-beta). Zenodo.). Zenodo https://zenodo.org/records/8013233 (2023).
Luke, D. R. Relaxed averaged alternating reflections for diffraction imaging. Inverse Probl. 21, 37–50 (2005).
Gerchberg, R. W. A practical algorithm for the determination of phase from image and diffraction plane pictures. Optik 35, 237–246 (1972).
Clark, J. N., Huang, X., Harder, R. & Robinson, I. K. High-resolution three-dimensional partially coherent diffraction imaging. Nat. Commun. 3, 993 (2012).
Favre-Nicolin, V., Leake, S. & Chushkin, Y. Free log-likelihood as an unbiased metric for coherent diffraction imaging. Sci. Rep. 10, 2664 (2020).
Acknowledgements
The authors thankfully acknowledge financial support from the European Union’s Horizon 2020 research and innovation funding program under grant agreements no. 851441 (SELECTCO2) and no. 101006701 (EcoFuel), as well as from the Deutsche Forschungsgemeinschaft (German Research Foundation, DFG) under grant no. STR 596/18–1. M.F. and A.A. thankfully acknowledge financial support by the Federal Ministry of Education and Research (Bundesministerium fuer Bildung und Forschung, BMBF) under grant no. 03SF0611A (H2Meer). L.W. gratefully acknowledges financial support from the Alexander von Humboldt Foundation. C.A. and M.-I.R. thankfully acknowledge financial support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 818823, CARINE). H.N.N. gratefully acknowledges funding from the DFG under grant no. STR 596/21-1 (DaCapo). We acknowledge the European Synchrotron Radiation Facility (ESRF) for provision of synchrotron radiation facilities using beamlines ID01 and ID16B. We also acknowledge DESY (Hamburg, Germany), a member of the Helmholtz Association HGF, for the provision of experimental facilities. Parts of this research were carried out at PETRA III using the P10 Coherence Applications Beamline. Beamtime was allocated for proposal I-20220139. We also thank Dipl.-Ing. Sören Selve and Dr. Christian Günther from the Zentraleinrichtung für Elektronenmikroskopie (ZELMI) at Technische Universitaet Berlin (TUB) for their support with FIB/SEM lamella preparation as well as STEM-EDX map**s. In addition, the authors thank Dr. Malte Klingenhof and Paul W. Buchheister (TUB) for their support with additional BCDI experiments carried out at the ID01 beamline at ESRF.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.F., L.W., C.A., M.-I.R., and P.S. designed the experiments and developed the concept of the study. M.F., L.W., M.H., R.T., L.L., J.L., and J.V. carried out in situ nano-XAS experiments, evaluated and discussed the data. M.F., L.W., C.A., Z.R., A.G., H.N.N., M.S., and M.-I.R. conducted in situ nano-BCDI experiments, evaluated and discussed the data. M.F. and L.W. carried out SEM and SEM/EDX analyses; M.F. and A.A. carried out TEM/SAED analysis after FIB/SEM lamella preparation and evaluated the data. L.W. performed the electrochemical tests of the eCO2RR catalysts. All authors contributed to scientific discussions and jointly revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frisch, M.L., Wu, L., Atlan, C. et al. Unraveling the synergistic effects of Cu-Ag tandem catalysts during electrochemical CO2 reduction using nanofocused X-ray probes. Nat Commun 14, 7833 (2023). https://doi.org/10.1038/s41467-023-43693-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43693-2
- Springer Nature Limited