Abstract
The biofabrication of three-dimensional (3D) tissues that recapitulate organ-specific architecture and function would benefit from temporal and spatial control of cell-cell interactions. Bioprinting, while potentially capable of achieving such control, is poorly suited to organoids with conserved cytoarchitectures that are susceptible to plastic deformation. Here, we develop a platform, termed Spatially Patterned Organoid Transfer (SPOT), consisting of an iron-oxide nanoparticle laden hydrogel and magnetized 3D printer to enable the controlled lifting, transport, and deposition of organoids. We identify cellulose nanofibers as both an ideal biomaterial for encasing organoids with magnetic nanoparticles and a shear-thinning, self-healing support hydrogel for maintaining the spatial positioning of organoids to facilitate the generation of assembloids. We leverage SPOT to create precisely arranged assembloids composed of human pluripotent stem cell-derived neural organoids and patient-derived glioma organoids. In doing so, we demonstrate the potential for the SPOT platform to construct assembloids which recapitulate key developmental processes and disease etiologies.
Similar content being viewed by others
Introduction
The development of the human nervous system is predicated upon spatiotemporally controlled interactions between cells from distinct lineages1. These interactions occur early in gestation and are therefore inherently inaccessible for studies that probe neurodevelopmental phenomena or evaluate the efficacy of drugs targeting tissues in their native environment. Human neural organoids, three-dimensional (3D) stem cell-derived cultures that self-organize and exhibit tissue-mimetic cytoarchitecture and physiology, have been shown to recapitulate facets of brain development in vitro2,3,4,5 and are beginning to reveal mechanistic insights into disease etiologies6,7. To model cell-cell interactions and circuit formation in the develo** brain, multiple neural organoids have been fused into single integrated tissues known as neural assembloids8,9,2. Given the imperative of a conserved cytoarchitecture within the neural organoid35,36, this degree of distension would be prohibitive to studies of neural development or disease.
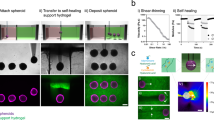
The SPOT platform relies upon the magnetic actuation of MNPs, which are embedded within a bioinert CNF ink biomaterial that encases the OBB. An iron rod affixed to an electromagnet mounted on a modified 3D printer is used to control the lifting, positioning, and deposition of the MNP-coated OBB. As such, with SPOT, the OBB is lifted in response to a force that is distributed across the entire OBB surface, unlike aspiration, which concentrates force and results in deformation during the lifting process (Fig. 1e, l). As a result, structural deformation with SPOT is not observed (Fig. 1m).
SPOT facilitates the controlled lifting, transfer, and deposition of neural organoids in 3D
The SPOT platform consists of the following series of repeatable, automatable steps: (i) coat organoids with the iron-oxide MNP embedded CNF ink, (ii) lift the coated organoid with an iron rod attached to an electromagnet-modified 3D printer, (iii) position the lifted organoid in 3D within a CNF support scaffold, and (iv) turn off the electromagnet and remove the iron rod (Fig. 2a, b).
a Schematic of the SPOT platform. b Representative images of a neural organoid being coated in a magnetic nanoparticle (MNP)-laden CNF ink, lifted and transferred into a CNF support scaffold by a magnetic rod attached to an electromagnet-modified 3D printer, and released at a desired position within the support scaffold. c Representative viscosity measurements of inks with 1 wt% MNP and various CNF wt%. d Quantification of the relative degree of MNP dispersion within CNF inks of various wt% over 15 minutes (n = 4 formulations); error bars represent standard deviation. Inset: Representative image of MNP dispersion within a microcentrifuge tube. e Representative image of MNP-laden CNF inks extruded over the top of neural organoids. f Representative storage modulus (filled circles) and loss modulus (open circles) of 0.5 wt% CNF support scaffold exposed to cyclical periods of low (0.1%) and high (300%) strain to evaluate the ability of the material to shear thin and self-heal. Percent G’ recovery following one cycle (mean ± SD): 86.8 ± 6.0 (n = 4 gels); percent G’ recovery following two cycles (mean ± SD): 84.3 ± 25.6 (n = 4 gels). g Storage modulus of CNF support scaffolds of various wt%. Each data point represents a distinct gel (n = 4 for all formulations). p values for storage moduli comparisons are as follows: 0.25 wt% CNF vs. 0.50 wt% CNF p = 0.9001, 0.50 wt% CNF vs. 1.00 wt% CNF p = 0.0097, 1.00 wt% CNF vs. 1.50 wt% CNF p < 0.0001, h Representative viscosity measurements of 0.5 wt% CNF support scaffolds in response to treatment with various concentrations of cellulase. i Quantification of the extent of MNP coverage on the surface of a neural organoid following coating with 1 wt% MNPs in DPBS or a 1 wt% MNP-laden 0.025 wt% CNF ink. Each data point represents a different organoid (n = 3). p values for MNP coverage comparisons are as follows: Pre-Lifting p = 0.048, Post-Release p = 0.0162, Post-Cellulase p = 0.851. j Representative BF image of a neural organoid following SPOT. Statistical analyses were performed as one-way ANOVA with Tukey multiple comparisons test or two-way ANOVA with either Dunnett’s multiple comparisons test or Šídák’s multiple comparisons test. Unless otherwise noted, all data points represent distinct biological replicates. Data plotted as mean ± SD where *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, and ns not significant.
The organoid coating process is achieved by first mixing MNPs into a CNF hydrogel and then dispensing fixed volumes of the mixture atop each individual OBB. Both commercially available MNPs and iron-oxide nanoparticles synthesized in-house through co-precipitation in a basic solution (Supplementary Table 2) were successfully utilized for OBB coating. After 30 minutes of coating, the MNPs are evenly distributed across the surface of the organoid (Supplementary Fig. 6a). To lift the coated organoid, a conventional 3D printer is modified such that an affixed electromagnet can be switched on and off with the same G-code that is used to direct the movement of the print head. An iron rod (here, with a diameter of 2 mm) is then bound to the electromagnet. Once the rod is bound, it is positioned above, and subsequently lowered toward, the coated organoid. As a function of the magnetic field strength, which is tuned by modulating the voltage of the electromagnet and the distance of the magnetic rod (Supplementary Fig. 7), the MNPs within the CNF ink are pulled towards the rod, resulting in lifting of the OBB (Supplementary Fig. 6b). As an alternative OBB coating approach that would be amenable to cultures grown within bioreactors, MNPs can be added directly to the medium of a suspension culture and agitated with an orbital shaker (Supplementary Fig. 8). Once the organoid is affixed to the end of the magnetized rod, it can be transported from the liquid medium into the CNF support scaffold. While the CNF support scaffold is directly adjacent to the organoids in the setup shown here (Figs. 2a, b), any configuration in which the organoids can be transferred while remaining submerged within cell culture medium is amenable to SPOT. Importantly, the final position of the organoid can be addressed in X, Y, and Z dimensions. Once the desired position is achieved, the electromagnet is turned off, and the iron rod is removed.
Characterization of the CNF ink and embedded MNPs
To support magnetic bioprinting, a potential cytocompatible ink material should: (i) undergo viscous thinning under an applied shear to allow for continuous dispensing of an MNP-laden ink through a syringe (ii) have a zero-shear viscosity that prevents MNP sedimentation over time scales relevant to coating multiple OBBs, (iii) encase the organoid fully once dispensed, and (iv) limit the degree of direct MNP contact with the organoid surface. A 0.025 percent by weight (wt%) solution of CNF exhibited a shear-thinning viscosity and significantly reduced MNP settling compared to 0.00 and 0.01 wt% CNF solutions (0.00 wt%: 64.1 ± 0.9% dispersed, 0.01 wt%: 64.4 ± 0.6% dispersed, 0.025 wt%: 82.0 ± 3.5% dispersed over 15 minutes, p < 0.0001; Figs. 2c, d). While the 0.10 wt% CNF ink solution resulted in significantly less MNP settling (97.3 ± 2.3% dispersed, p < 0.0001), it did not adequately encase the organoid due to its higher viscosity and, therefore, was less well suited to reproducible organoid lifting (Fig. 2e). Following 30 minutes of incubation, the 0.025 wt% CNF ink uniformly coated the organoid with MNPs (Supplementary Figs. 6a and 9a). Qualitative evaluation of potential MNP uptake using Prussian blue staining of OBB cross-sectional slices demonstrated that the MNPs were primarily located at the periphery of the organoid without extensive intracellular localization (Supplementary Fig. 9b). While iron was detected throughout the coated organoid, a similar distribution was observed in control neural organoids that had never been exposed to MNPs (Supplementary Fig. 9c). Although iron oxide nanoparticles were previously shown to affect the MAPK signaling pathway in bone-derived MSCs37, MAPK signaling in hiPSC-derived dorsal forebrain organoids was not affected by MNP surface coverage with SPOT (Supplementary Fig. 9d).
Characterization of the CNF support scaffold and organoid release
In biofabrication, support scaffolds temporarily maintain the spatial positioning of cells within 3D space. To achieve this, support scaffolds must: (i) be shear-thinning so that the material yields as a deposition tool moves through the scaffold, and (ii) be self-healing after the deposition tool has passed to provide physical confinement to the OBB29,38. To create an optimal support scaffold, we sought to identify a material that was cytocompatible, bioinert to mammalian cells, and amenable to on-demand solubilization to release the encapsulated cellular structure after fabrication.
We evaluated the viscoelastic properties of a range of CNF solutions for use as a support scaffold. A concentration of 0.50 wt% CNF exhibited shear-thinning and self-healing properties without the need for additional chemical modifications or formulation additives (Fig. 2f). Moreover, the 0.50 wt% CNF demonstrated a greater recovery of modulus after high strain compared to 0.25 wt%, 1.0 wt%, or 1.5 wt% CNF (Fig. 2f and Supplementary Fig. 10). The reported stiffness of neural tissue varies as a function of sample age, brain region, and testing method, yet most studies report shear moduli ranging from several hundred to a few thousand pascal (Pa)39,40,41. The wt% of CNF can be tuned to reproducibly vary the stiffness of support, and the 0.5 wt% CNF had a plateau storage modulus (G’) of approximately 150 Pa (Fig. 2g).
After being positioned, the fusion of constituent OBBs into a single assembloid can require multiple days during which the support scaffold should remain intact. The 0.5 wt% CNF support scaffold displayed a consistent range of storage moduli over 72 h, both with and without daily media changes (Supplementary Fig. 11a). Furthermore, the 0.5 wt% CNF support scaffold allowed for consistent media diffusion (Supplementary Table 3). After OBB fusion, the resultant assembloid can be removed from the scaffold for downstream applications. Although recent efforts have begun to introduce polymers into the medium of regionalized neural organoids42, to date, most studies have cultured organoids in suspension without the addition of exogenous biomaterials36. CNF, as a cellulose derivative, is amenable to cellulase-mediated degradation (Supplementary Fig. 11b). Treating the CNF support with a range of cellulase concentrations resulted in stepwise decreases in both viscosity and storage modulus over time (Fig. 2h and Supplementary Fig. 11c, d). Importantly, as cellulase activity is bioorthogonal to mammalian cultures, the addition of cellulase does not affect organoid viability (Supplementary Fig. 11e). Once released from the CNF support bath with 0.5 wt% cellulase, neural organoids may remain sparsely coated in residual CNF; however, the addition of 0.5 wt% cellulase removes over 98% of the material over 72 hours (Supplementary Fig. 12). In lieu of cellulase-mediated degradation, organoids can be released from their support scaffold through the gentle dilution of the CNF hydrogel with DPBS. Alternatively, organoids may be cultured within the scaffold for protracted periods of time.
Following the entire coating, lifting, transportation, deposition, and removal process, the majority of MNPs located on the surface of the neural organoids were no longer present (Supplementary Fig. 13). Moreover, by initially coating an organoid with an MNP-laden CNF bioink, as opposed to MNPs in solution, the degree of MNP attachment to the organoid surface significantly decreased (prior to lifting: p < 0.05 between MNP and MNP in CNF, post release from CNF: p < 0.05 between MNP and MNP in CNF, post cellulase treatment: p = 0.851) (Fig. 2i). Finally, following SPOT, the neural organoids appear devoid of the gross deformations observed with AAB (Fig. 2j).
Utilizing SPOT to construct dorsal-ventral forebrain assembloids
The construction of multi-region neural assembloids that begin to recapitulate the circuitry of the develo** brain was first demonstrated in a collection of studies in 20178,9,28,77,78. Briefly, a clear, plastic, blunt edge nozzle on a syringe was affixed to a DBPS-containing 35 mm plate. The syringe was connected to a pressure modulator, which was connected to a vacuum line. A range of pressures (∆P = 1–30 mmHg) was applied to the OBB surface, and the subsequent deformation was observed on an epifluorescent microscope (Leica Microsystems, THUNDER Imager 3D Cell Culture).
Vacuum aspiration
OBBs were exposed to vacuum aspiration pressures following the same protocol described above for apparent surface tension measurements. Importantly, to ensure consistent pressure was applied to a given OBB, the desired pressure was first reproducibly obtained in DPBS before the blunt edge nozzle was lowered to the surface of the OBB.
Neural organoid viability
To characterize viability, organoids were submerged in a solution consisting of DPBS supplemented with 2 µM calcein AM and 4 µM ethidium homodimer for 20 min at 37 °C (Thermo Fisher Scientific L3224). The samples were washed with DPBS and imaged with an epifluorescent microscope (Leica Microsystems, THUNDER Imager 3D Cell Culture) and confocal microscope (Leica SPE).
MNP fabrication
Iron oxide magnetic particles were fabricated in-house by the conventional method of co-precipitation, in which ferrous and ferric ions are mixed in a 1:2 molar ratio in a basic solution79. Briefly, 0.05 M iron(II)sulfate (Sigma-Aldrich F7002) and 0.1 M iron(III)chloride (Sigma-Aldrich 157740) were first dissolved together in water at room temperature. A solution of 10% ammonium hydroxide was added to the reaction dropwise through a separatory funnel with constant stirring (500 rpm) for 1 h. Following the completion of the reaction, the iron oxide particles were washed three times with water.
Commercial iron oxide nanoparticles were purchased from Alpha Nanotech Inc. (size: 300 nm, surface coating: polydopamine coating).
MNP size distribution and zeta potential
To determine the hydrodynamic size distribution, dynamic light scattering was performed on a 0.1 wt% MNP in DPBS solution using a Malvern Zetasizer Nano ZS. To determine the distribution of aggregate sizes, a 0.1 wt% MNP in DPBS solution was sonicated in a bath sonicator for 5 min, sandwiched between two glass slides, imaged with a Leica THUNDER microscope, and processed with FIJI. To determine the zeta potential, a 0.01 wt% MNP in DPBS solution was sonicated in a bath sonicator for 5 minutes, suspended in folded capillary cells (Malvern DTS1060), and characterized, with 10 runs per sample, in a Malvern Zetasizer Nano ZS.
MNP surface coverage
To quantify organoid surface MNP coverage, brightfield Z-stack images were taken using a Leica THUNDER microscope. Background subtraction was performed using the rolling ball algorithm (radius = 25 pixels), and MNP coverage area was measured via thresholding and maximum Z projection.
Magnetic field strength characterization
To characterize the magnetic field applied during lifting, magnetic field strength measurements were performed as a function of applied voltage and distance from the probe tip using a LATNEX MK-30K AC/DC Gauss meter and electromagnet-modified Prusa i3 MK3S 3D printer.
CNF fabrication
Stock solutions of bacterial CNF for the magnetic ink and support scaffold were fabricated from nata de coco (Jubes). The nata de coco cubes were washed with flowing deionized water for two days. Following the washes, coco de nata and deionized water were blended together in a 1:1 ratio until homogenous. The solution was concentrated through centrifugation at 12857×g for 20 minutes, autoclaved for sterilization, and stored at 4 °C. To calculate the concentration of the CNF stock solution, aliquots were weighed before and after drying. For use in the magnetic ink or support scaffold, the CNF stock was diluted with sterile DPBS.
CNF macrorheological characterization
Mechanical testing of the CNF-based magnetic ink and support scaffold formulations was performed using an AR-G2 (TA Instruments) stress-controlled rheometer (8 mm and 40 mm parallel plate geometry with a 1 mm gap) at 25 °C. For the storage and loss moduli, frequency sweeps were performed between 0.1 and 100 rad s−1 at a strain of 1%, and measurements were confirmed to be within the linear viscoelastic regime. Viscosity tests were performed at shear rates ranging from 0.1 to 10 s−1. For self-healing measurements, alternating strains of 0.1% and 300% were applied.
Magnetic lifting
OBBs were coated with 10 μL of a magnetic ink composed of 1 wt% MNPs in 0.025 wt% CNF for 30 min. Magnetic rods were affixed to an electromagnet set to 15 V. The rod was then lowered until it was just above the surface of the coated OBB. Once the OBB attached to the rod, the entire construct was moved throughout DPBS to emulate the movement an OBB would experience in the printing process.
Bioprinting chip design and fabrication
To facilitate automated magnetic bioprinting, we designed a chip that ensured that a series of OBBs were consistently located at a given position. Additional features included an offset platform for medium addition, a row of elongated U-bottom wells, and a raised connector channel between the wells and the reservoir which contained the support scaffold. The chip was created by pouring an uncured mixture of Sylgard 184 (Dow Corning 2646340) in 10:1 base to curing agent ratio into a 3D printed polylactic acid mold. The PDMS was degassed under vacuum for 20 min and cured at room temperature (RT) for 48 h before being carefully removed from the mold.
Automated magnetic bioprinting
Automated transfer was achieved using an electromagnet-modified Monoprice MP Select Mini 3D Printer V2 and Kaiweets PS-3010F DC power supply. The printer was modified such that the print-head fan controls were wired, via a solid-state relay, to the power supply which, in turn, activated an electromagnet. As the fan can be turned on or off with G-Code, the electromagnet itself was controllable with the same code that was used to address the movement of the OBB.
Precision of automated magnetic bioprinting
For the measurement of XY localization, alginate beads with diameters comparable to those of neural organoids (1–2 mm) were coated with a magnetic ink composed of 1 wt% MNPs in 0.025 wt% CNF for 30 min and transferred from water to a pre-specified location within a 0.5 wt% CNF bath using the magnetic 3D bioprinting approach. ImageJ (NIH, v.2.3.0/1.53q) was used to measure the XY deviation of the deposited alginate bead from the marked position.
To measure bioprinting precision in Z, alginate beads were transferred from water into a 0.5 wt% CNF support bath with the magnetic rod. The Z position of the bead was tracked via imaging over 3 days.
CNF support diffusivity characterization
The diffusivity within the CNF support scaffold was assessed by fluorescent recovery after photobleaching (FRAP) measurements. Briefly, 0.5 wt% CNF was prepared with encapsulated FITC-dextran probes (Sigma) with molecular weights of 10 kDa, 20 kDa, 40 kDa, 70 kDa, 150 kDa, 250 kDa, and 500 kDa. The FRAP experiments were then performed on a confocal microscope (Leica SPE) with 1 min of photobleaching (100 μm x 100 μm bleach area, 488 nm laser, 100% intensity) followed by 4 min of capture time (10% intensity). The diffusion coefficients for each probe size were determined using the open-source MATLAB code “frap_analysis” based on the Hankel transform method80.
Cellulase-mediated CNF scaffold degradation
For measurements of cellulase-mediated CNF support scaffold degradation, cellulase (Sigma-Aldrich C1794) was dissolved in DMEM/F12 (Thermo Fisher Scientific 11320033) and added atop 0.5 wt% CNF in a 1:4 ratio by volume to approximate the ratio of media to CNF support within the bioprinting chip. All samples were incubated in a humidified 37 °C incubator for 3 days. Depending on the downstream measurement, cellulase solutions, as well as non-cellulase containing DMEM/F12 controls, were either changed every day or allowed to incubate over the full three days without a media change.
Viscosity and storage moduli were obtained with an AR-G2 (TA Instruments) stress-controlled rheometer (20 mm 1° cone and plate geometry with a 28 μm gap). To observe the effect of cellulase on the viscosity of CNF over 72 h, samples were loaded onto the stage at 37 °C for a 5 min time sweep with 1% oscillatory strain and 1 rad/s angular frequency. This was followed by a frequency sweep from 0.1 to 100 Hz at 1% strain. To measure the storage modulus, samples were loaded onto the stage at 2 h, 4 h, 6 h, 24 h, 48 h, and 72 h and subjected to a frequency sweep at 1 rad/s angular frequency.
Cellulase-mediated degradation of CNF surrounding extracted neural organoids
To characterize cellulase-mediated CNF degradation after neural organoid release from the support scaffold, neural organoids were first cultured in 0.5 wt% CNF support bath for 1 day and released through diluting the support bath with DPBS. A 0.5 wt% cellulase (Sigma) solution, made up in neural medium, was filtered through a 0.22 μm filter, warmed to 37 °C, and added to the organoids with daily medium changes. Organoids were imaged every day with an epifluorescent microscope (Leica Microsystems, THUNDER Imager 3D Cell Culture). The area of residual CNF on the organoid surface was manually measured with ImageJ (NIH, v.2.3.0/1.53q).
Quantitative reverse transcription polymerase chain reaction
mRNA expression was quantified with quantitative reverse transcription polymerase chain reaction. Organoids were suspended in 500 μL of TRIzol reagent (Thermo Fisher Scientific 15596026) and disrupted via probe sonication (Heilscher UP50H, 50% amplitude (25 watts), 30 kHz frequency, 0.5 cycle). mRNA was purified by phenol-chloroform extraction with phase lock gels (Quantabio 5PRIME 2302830) followed by isopropyl alcohol precipitation. The resultant mRNA was resuspended in nuclease-free water (Thermo Fisher Scientific 10977015) and measured via NanoDrop (Thermo Fisher Scientific). 100 ng of mRNA was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems 4368814). For qPCR, 6.6 μL of diluted cDNA was mixed with 0.9 μL of a 5 μM forward and reverse primer pair (Integrated DNA Technologies, Supplementary Table 3) solution and 7.5 μL of Fast SYBR Green Master Mix (Applied Biosystems 4385612). Samples were run on a StepOnePlus Real Time PCR System (Applied Biosystems). CT values were calculated using the StepOnePlus software (v.2.3) and analyzed by the ΔCT method.
Panobinostat treatment of neuro-DIPG assembloids
Neuro-DIPG assembloids fabricated with SPOT fused within 24 h. They were subsequently released from the CNF support bath using cellulase treatment as described above and cultured for one week in suspension, with media changes performed every 3-4 days. After 1 week in suspension culture, the media was replaced with fresh media containing 200 nM panobinostat (Selleckchem S1030). Neuro-DIPG assembloids were cultured in the presence of 200 nM panobinostat for 72 h, with no media changes, after which samples were fixed in 4% paraformaldehyde (PFA, Electron Microscopy Sciences 15700) in DPBS for immunohistochemistry. Control samples were also given fresh media without panobinostat on day 7 and cultured for 72 h.
Immunohistochemistry
Organoids and assembloids were fixed in 4% PFA for 2 h at 4 °C. They were then washed three times with DPBS, for 15 min each, and transferred to a 30% sucrose solution in DPBS for 24–48 h at 4 °C. Once the organoids or assembloids sank in the sucrose solution, they were embedded in a 1:1 mixture of OCT (Fisher Scientific 23-730-571) and 30% sucrose in DPBS. They were then snap frozen on dry ice and stored at −80 °C. A cryostat (Leica) was used to cut 50 µm sections for immunostaining.
Cryosections were washed with DPBS to remove excess OCT, then permeabilized with 0.25% Triton X-100 (Thermo Fisher Scientific A16046) in DPBS (DPBS-T) for 1 h and blocked with 5% goat serum (Gibco 16210-072), 5% bovine serum albumin (BSA, Sigma A9418), and 0.5% Triton X-100 in DPBS for 3 hours, all at RT. Samples were stained with primary antibodies for GFP, (1:200, Thermo Fisher Scientific a11122), cleaved caspase-3 (1:400, Cell Signaling 9661), histone H3 mutated K27M (1:400, Abcam ab190631), paired box 6 (1:200, Biolegend 901301), NK2 homeobox 1 (1:100, Thermo Fisher Scientific ma5-13961), beta-tubulin 3 (1:500, Aves Labs TUJ), and GFAP (1:500, Aves Labs GFAP). Primary antibodies were diluted in 2.5% goat serum, 2.5% BSA, and 0.5% Triton X-100 in DPBS and incubated with the samples overnight at 4 °C. Next, the samples were washed with DPBS-T (3 × 30 min, RT) and incubated with secondary antibodies Alexa Fluor 488 (1:500, Thermo Fisher Scientific A-11034), Alexa Fluor 594 (1:500, Thermo Fisher Scientific A-11020), Alexa Fluor 633 (1:500, Thermo Fisher Scientific A-21103), and 4′,6-diamidino-2-phenylindole (DAPI, 5 mg/mL stock, 1:2000, Thermo Fisher Scientific 62247) in the same antibody dilution solution overnight at 4 °C. Finally, the samples were washed with DPBS-T (3 × 20 min, RT) and mounted to No. 1 glass cover slips with ProLong Gold Antifade Reagent (Cell Signaling 9071). Stained samples were imaged using a confocal microscope (Leica SPE) and process with Las-X software (Leica).
Image analysis
Cleaved caspase-3 expression and H3K27M expression were analyzed from maximum projection immunofluorescence images using CellProfiler81. Images were cropped such that only the DIPG organoid area was included in analysis. For each cell, nuclei and H3K27M objects were identified using the “IdentifyPrimaryObjects” command with a “Minimum Cross-Entropy” thresholding method. Cleaved caspase-3 objects were identified using the “IdentifyPrimaryObjects” command with an “Otsu” thresholding method. Overall area of expression was obtained using the “MeasureImageAreaOccupied” command. Cleaved caspase-3 expression and H3K27M expression were normalized by DAPI count and then normalized to the untreated control.
Statistical analysis and reproducibility
Statistical analyses for this study were performed using GraphPad Prism v.9.3.1 software. Details of specific statistical methods and p-value results are included within the figure captions and summarized in Supplementary Data 1. For all studies, ns = not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
All representative images of neural organoids were obtained from four independent differentiation experiments with similar results. Images associated with aspiration, deformation, and organoid lifting were obtained from at least four independent repetitions. Images associated with organoid printing, fusion, and integration were obtained from at least ten independent repetitions. Images associated with tumor infiltration and drug treatment were obtained from at least three independent repetitions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the results reported in this manuscript are available in the Stanford Digital Repository under the persistent https://purl.stanford.edu/sw198jy9339 and the https://doi.org/10.25740/sw198jy9339.
Code availability
All G-code central to the use of SPOT for organoid bioprinting is included as supplementary information.
References
Kelley, K. W. & Pașca, S. P. Human brain organogenesis: toward a cellular understanding of development and disease. Cell 185, 42–61 (2022).
Pasca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Sloan, S. A. et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790.e776 (2017).
Marton, R. M. et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 22, 484–491 (2019).
Gordon, A. et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 24, 331–342 (2021).
Pașca, A. M. et al. Human 3D cellular model of hypoxic brain injury of prematurity. Nat. Med. 25, 784–791 (2019).
Khan, T. A. et al. Neuronal defects in a human cellular model of 22q11.2 deletion syndrome. Nat. Med. 26, 1888–1898 (2020).
Birey, F. et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54 (2017).
Bagley, J. A., Reumann, D., Bian, S., Lévi-Strauss, J. & Knoblich, J. A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751 (2017).
**ang, Y. et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398.e387 (2017).
Miura, Y. et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol. 38, 1421–1430 (2020).
Andersen, J. et al. Generation of functional human 3D cortico-motor assembloids. Cell 183, 1913–1929.e1926 (2020).
Kasai, T. et al. Hypothalamic contribution to pituitary functions is recapitulated in vitro using 3D-cultured human iPS cells. Cell Rep. 30, 18–24.e15 (2020).
Fligor, C. M. et al. Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Rep. 16, 2228–2241 (2021).
Birey, F. et al. Dissecting the molecular basis of human interneuron migration in forebrain assembloids from Timothy syndrome. Cell Stem Cell 29, 248–264.e247 (2022).
Miura, Y. et al. Engineering brain assembloids to interrogate human neural circuits. Nat. Protoc. 17, 15–35 (2022).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
LeSavage, B. L., Suhar, R. A., Broguiere, N., Lutolf, M. P. & Heilshorn, S. C. Next-generation cancer organoids. Nat. Mater. 21, 143–159 (2022).
Boland, T., Mironov, V., Gutowska, A., Roth, E. A. & Markwald, R. R. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 272, 497–502 (2003).
Jakab, K., Neagu, A., Mironov, V., Markwald, R. R. & Forgacs, G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc. Natl Acad. Sci. USA 101, 2864–2869 (2004).
Jakab, K. et al. Tissue engineering by self-assembly of cells printed into topologically defined structures. Tissue Eng. Part A 14, 413–421 (2008).
Norotte, C., Marga, F. S., Niklason, L. E. & Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30, 5910–5917 (2009).
Skardal, A., Zhang, J. & Prestwich, G. D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 31, 6173–6181 (2010).
Mironov, V. et al. Organ printing: tissue spheroids as building blocks. Biomaterials 30, 2164–2174 (2009).
Wolf, K. J., Weiss, J. D., Uzel, S. G. M., Skylar-Scott, M. A. & Lewis, J. A. Biomanufacturing human tissues via organ building blocks. Cell Stem Cell 29, 667–677 (2022).
Goulart, E. et al. 3D bioprinting of liver spheroids derived from human induced pluripotent stem cells sustain liver function and viability in vitro. Biofabrication 12, 015010 (2019).
Skylar-Scott, M. A. et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 5, eaaw2459 (2019).
Ayan, B. et al. Aspiration-assisted bioprinting for precise positioning of biologics. Scie. Adv. 6, eaaw5111 (2020).
Daly, A. C., Davidson, M. D. & Burdick, J. A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 12, 753 (2021).
Ayan, B. et al. Aspiration-assisted freeform bioprinting of pre-fabricated tissue spheroids in a yield-stress gel. Commun. Phys. 3, 183 (2020).
Kim, M. H., Banerjee, D., Celik, N. & Ozbolat, I. T. Aspiration-assisted freeform bioprinting of mesenchymal stem cell spheroids within alginate microgels. Biofabrication 14, 024103 (2022).
Sloan, S. A., Andersen, J., Pașca, A. M., Birey, F. & Pașca, S. P. Generation and assembly of human brain region–specific three-dimensional cultures. Nat. Protoc. 13, 2062–2085 (2018).
Hochmuth, R. M. Micropipette aspiration of living cells. J Biomech 33, 15–22 (2000).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Albanese, A. et al. Multiscale 3D phenoty** of human cerebral organoids. Sci. Rep. 10, 21487 (2020).
Roth, J. G. et al. Advancing models of neural development with biomaterials. Nat. Rev. Neurosci. 22, 593–615 (2021).
Wang, Q. et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 86, 11–20 (2016).
Brunel, L. G., Hull, S. M. & Heilshorn, S. C. Engineered assistive materials for 3D bioprinting: support baths and sacrificial inks. Biofabrication 14, 032001 (2022).
Gefen, A. & Margulies, S. S. Are in vivo and in situ brain tissues mechanically similar? J. Biomech. 37, 1339–1352 (2004).
Elkin, B. S., Azeloglu, E. U., Costa, K. D. & Morrison, B. 3rd. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J. Neurotrauma 24, 812–822 (2007).
Budday, S. et al. Rheological characterization of human brain tissue. Acta Biomater. 60, 315–329 (2017).
Narazaki, G. et al. Biocompatible polymers for scalable production of human neural organoids. bioRxiv (2022).
Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 13, 107–120 (2012).
Fisher, P. G. et al. A clinicopathologic reappraisal of brain stem tumor classification. Cancer 89, 1569–1576 (2000).
Johung, T. B. & Monje, M. Diffuse intrinsic pontine glioma: new pathophysiological insights and emerging therapeutic targets. Curr. Neuropharmacol. 15, 88–97 (2017).
Sturm, D. et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat. Rev. Cancer 14, 92–107 (2014).
Jones, C. & Baker, S. J. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat. Rev. Cancer 14, 651–661 (2014).
Lin, G. L. et al. Non-inflammatory tumor microenvironment of diffuse intrinsic pontine glioma. Acta Neuropathol. Commun. 6, 51 (2018).
Yoshimura, J., Onda, K., Tanaka, R. & Takahashi, H. Clinicopathological study of diffuse type brainstem gliomas: analysis of 40 autopsy cases. Neurol. Med. Chir. 43, 375–382 (2003)
Gururangan, S. et al. Incidence and patterns of neuraxis metastases in children with diffuse pontine glioma. J. Neurooncol. 77, 207–212 (2006).
Caretti, V. et al. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol. 128, 605–607 (2014).
Lin, G. L. & Monje, M. A protocol for rapid post-mortem cell culture of diffuse intrinsic pontine glioma (DIPG). J. Vis. Exp. 121, e55360 (2017).
Grasso, C. S. et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat. Med. 21, 555–559 (2015).
Puget, S. et al. Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Childs Nerv. Syst. 31, 1773–1780 (2015).
Lin, G. L. et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci. Transl. Med. 11, eaaw0064 (2019).
Qin, E. Y. et al. Neural precursor-derived pleiotrophin mediates subventricular zone invasion by glioma. Cell 170, 845–859.e819 (2017).
Louis, D. N. et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820 (2016).
Filbin, M. & Monje, M. Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat. Med. 25, 367–376 (2019).
Misuraca, K. L., Cordero, F. J. & Becher, O. J. Pre-clinical models of diffuse intrinsic pontine glioma. Front. Oncol. 5, 172 (2015).
Welby, J. P. et al. Current murine models and new developments in H3K27M diffuse midline gliomas. Front. Oncol. 9, 92 (2019).
Wu, G. et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44, 251–253 (2012).
Lewis, P. W. et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340, 857–861 (2013).
Bender, S. et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24, 660–672 (2013).
Chan, K. M. et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev 27, 985–990 (2013).
Funato, K., Major, T., Lewis, P. W., Allis, C. D. & Tabar, V. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27M histone mutation. Science 346, 1529–1533 (2014).
Piunti, A. et al. Therapeutic targeting of polycomb and BET bromodomain proteins in diffuse intrinsic pontine gliomas. Nat. Med. 23, 493–500 (2017).
Stafford, J. M. et al. Multiple modes of PRC2 inhibition elicit global chromatin alterations in H3K27M pediatric glioma. Sci. Adv. 4, eaau5935 (2018).
Harutyunyan, A. S. et al. H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat. Commun. 10, 1262 (2019).
Yang, Q. & Liberali, P. Collective behaviours in organoids. Curr. Opin. Cell Biol. 72, 81–90 (2021).
Moldovan, N. I., Hibino, N. & Nakayama, K. Principles of the kenzan method for robotic cell spheroid-based three-dimensional bioprinting. Tissue Eng. Part B Rev. 23, 237–244 (2017).
Souza, G. R. et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 5, 291–296 (2010).
Haisler, W. L. et al. Three-dimensional cell culturing by magnetic levitation. Nat. Protoc. 8, 1940–1949 (2013).
Roth, J. G. et al. 16p11.2 microdeletion imparts transcriptional alterations in human iPSC-derived models of early neural development. Elife 9, e58178 (2020).
Ang, L. T. et al. Generating human artery and vein cells from pluripotent stem cells highlights the arterial tropism of Nipah and Hendra viruses. Cell 185, 2523–2541.e2530 (2022).
Yoon, S. J. et al. Reliability of human cortical organoid generation. Nat. Methods 16, 75–78 (2019).
Nagaraja, S. et al. Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell 31, 635–652.e636 (2017).
Heinrich, V., Leung, A. & Evans, E. Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. II. Tether flow terminated by P-selectin dissociation from PSGL-1. Biophys. J. 88, 2299–2308 (2005).
Guevorkian, K., Colbert, M. J., Durth, M., Dufour, S. & Brochard-Wyart, F. Aspiration of biological viscoelastic drops. Phys. Rev. Lett. 104, 218101 (2010).
Wu, W., He, Q. & Jiang, C. Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nanoscale Res. Lett. 3, 397–415 (2008).
Jönsson, P., Jonsson, M. P., Tegenfeldt, J. O. & Höök, F. A method improving the accuracy of fluorescence recovery after photobleaching analysis. Biophys. J. 95, 5334–5348 (2008).
McQuin, C. et al. CellProfiler 3.0: next-generation image processing for biology. PLoS Biol. 16, e2005970 (2018).
Acknowledgements
The authors would like to acknowledge Bauer LeSavage for discussion and neural organoid maintenance, Riley Suhar for assistance with assembloid sectioning and immunohistochemistry, Rameshwar Rao for discussion, Ji-il Kim for assistance with assembloid sectioning, Jonas Fowler, Kyle Loh, Fabian Suchy, Joydeep Bhadury, and Hiro Nakauchi for the derivation and genetic manipulation of the hiPSC lines, Jared Hysinger and Michelle Monje for providing the DIPG cells, and patients and their families for their generous donations.
This work was facilitated by support from the Wu Tsai Neurosciences Brain Organogenesis Project, the National Institutes of Health (R01 EB027171, S.C.H.), and the National Science Foundation (NSF) (DMR 2103812, CBET 2033302, S.C.H.).
J.G.R. acknowledges support from the NSF Graduate Research Fellowship Program (GRFP) (DGE-1656518) and the Stanford Smith Family Graduate Fellowship. L.G.B. acknowledges support from the NSF GRFP (DGE-1656518). M.S.H. acknowledges support from the NSF GRFP (DGE-1656518) and the Stanford ChEM-H O’Leary-Thiry Graduate Fellowship.
Author information
Authors and Affiliations
Contributions
J.G.R. designed the research, conducted experiments, analyzed the data, assembled the data into figures, and wrote the manuscript. L.G.B. assisted with the design and execution of experiments pertaining to biomaterials synthesis and characterization as well as bioprinting accuracy. M.S.H. assisted with the design and execution of experiments pertaining to organoid fusion, bioprinting accuracy, and drug efficacy. Y.L. assisted with the design and execution of experiments pertaining to enzymatic degradation of the biomaterial scaffold. B.C. assisted with the design and execution of experiments pertaining to bioprinting accuracy and organoid coating with nanoparticles. Sa.S. assisted with the design and execution of experiments pertaining to DIPG fusion and drug treatment. Su.S. assisted with the design and execution of experiments pertaining to biomaterials synthesis, characterization, bioprinting accuracy and feasibility, and early efforts to automate the platform. F.Y., S.P.P., and S.C.H. provided guidance on the project and interpretation of data.
Corresponding author
Ethics declarations
Competing interests
J.G.R., L.G.B., Su.S., and S.C.H. are inventors on a patent application (no. 63/337,794) submitted by the Board of Trustees of Stanford University. The authors declare no other competing interests.
Peer review
Peer review information
Nature Communications thanks Amin Shavandi and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roth, J.G., Brunel, L.G., Huang, M.S. et al. Spatially controlled construction of assembloids using bioprinting. Nat Commun 14, 4346 (2023). https://doi.org/10.1038/s41467-023-40006-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40006-5
- Springer Nature Limited
This article is cited by
-
Laminin-associated integrins mediate Diffuse Intrinsic Pontine Glioma infiltration and therapy response within a neural assembloid model
Acta Neuropathologica Communications (2024)
-
Integrating organoids and organ-on-a-chip devices
Nature Reviews Bioengineering (2024)
-
Engineering brain-on-a-chip platforms
Nature Reviews Bioengineering (2024)
-
Biofabrication of Neural Organoids: An Experiential Learning Approach for Instructional Laboratories
Biomedical Engineering Education (2024)
-
Erzeugung menschlicher Nervengewebe in der Kulturschale
BIOspektrum (2023)